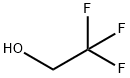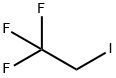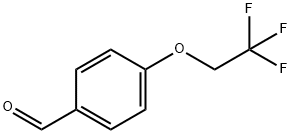
4-(2,2,2-trifluoroethoxy)benzaldehyde synthesis
- Product Name:4-(2,2,2-trifluoroethoxy)benzaldehyde
- CAS Number:76579-46-9
- Molecular formula:C9H7F3O2
- Molecular Weight:204.15
Yield:76579-46-9 90%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 80; for 18 h;
Steps:
25.A; A69.A Part A
To a solution of 4-fluoro-benzaldehyde (1) (Aldrich, 8.0 g, 64.4 mmol) in N,N-dimethylformamide (120 ml) was added potassium carbonate (Aldrich, 13.4 g, 96.7 mmol) followed by 3,3,3-trifluoroethanol (2) (6.4 g, 64.4 mmol). The mixture was stirred at 80° C. for 18 hr. After cooling to room temperature, the mixture was diluted with water, and the resulting solid was filtered. The filter cake was washed with water and dried in vacuo to afford compound (3) as a white solid (12.5 g, 95% yield). 1H NMR indicated the desired compound (3). A round bottom flask was charged with 4-fluorobenzaldehyde (25 g, 202 mmol, Aldrich) and 3,3,3-trifluoroethanol (22.2 g, 222 mmol, Aldrich) in dimethylformamide (400 ml). Potassium carbonate (41.7 g, 303 mmol, Aldrich) was added, the reaction was heated to 80° C., and stirred for 18 hr. The reaction was diluted with water (500ml) precipitating a white solid. The solid was collected by filtration, washed with water, and then dried to afford the product as a white solid (37 g, 90 % yield). 1H NMR showed the desired compound.
References:
US2005/9838,2005,A1 Location in patent:Page 145; 350
433-06-7
207 suppliers
$9.00/1g

76579-46-9
18 suppliers
inquiry