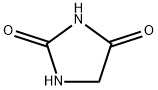
4-[(2,5-DIOXOIMIDAZOLIDIN-1-YL)METHYL]BENZONITRILE synthesis
- Product Name:4-[(2,5-DIOXOIMIDAZOLIDIN-1-YL)METHYL]BENZONITRILE
- CAS Number:565174-36-9
- Molecular formula:C11H9N3O2
- Molecular Weight:215.2081
Yield:565174-36-9 46.8%
Reaction Conditions:
Stage #1: 2,4-imidazolidinedionewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
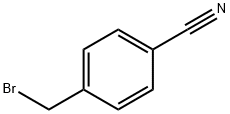
Stage #2: 4-cyanobenzyl bromidewith tetra-(n-butyl)ammonium iodide in N,N-dimethyl-formamide at 20; for 12 h;
Steps:
Intermediate 6: 4-((2,5-Dioxoimidazolidin-l-yl)methyl)benzonitrile (F-l)
To a stirred solution of imidazolidine-2,4-dione (3.0 g, 29.9 mmol) in 30 mL of DMF was added portion wise NaH (0.72 g, 29.9 mmol) at 0 °C. The reaction mixture was stirred for 30 min at 0 °C followed by addition of TBAI (0.1 g, 16.4 mmol) and 4- (bromomethyl)benzonitrile (5.8 g, 29.9 mmol) . The reaction mixture was stirred for 12 h at RT followed by diluting with water and extracting with ethyl acetate. The combined extracts were washed with water and brine, dried over anhydrous sodium sulphate and concentrated to give the title compound (3.0 g, 46.8 %). JH NMR (300 MHz, DMSO-dg) d 8.2 (s, -1H), 7.82- 7.80 (dd, 1H), 7.46 - 7.43 (dd, 2H), 4.68 (s, 2H), 4.08 (s, 2H).
References:
WO2019/180628,2019,A1 Location in patent:Page/Page column 63-64