
4-(2-BroMoethoxy)-4'-hydroxybenzophenone synthesis
- Product Name:4-(2-BroMoethoxy)-4'-hydroxybenzophenone
- CAS Number:79578-62-4
- Molecular formula:C15H13BrO3
- Molecular Weight:321.17
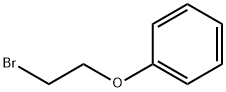
589-10-6
268 suppliers
$18.00/5g
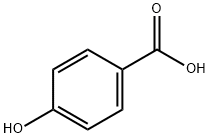
99-96-7
808 suppliers
$5.00/10g

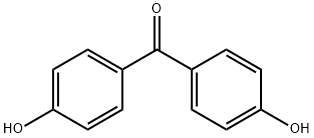
79578-62-4
10 suppliers
$1320.00/1g
Yield:79578-62-4 85%
Reaction Conditions:
with phosphorus tribromide;zinc dibromide at 40 - 50; for 19.5 h;Large scale;
Steps:
2 EXAMPLE 2. SYNTHESIS OF (4-(2-BROMOETHOXY)PHENYL)(4-HYDROXYPHENYL)METHANONE
Zinc bromide (3.011 kg, 13.1 mol) and polyphosphoric acid (16.5 kg) were charged into a 20-L reaction vessel and stirred well while heated to 45°C. 4-Hydroxybenzoic acid (974 g, 6.98 mol) and 2-phenoxyethyl bromide (1302 g, 6.34 mol) were added Phosphorus tribromide (2.72 kg, 9.83 mol) was added via an addition funnel over a period of 2.5 hours at a rate as to keep temperature below 50°C. Stirring at 40 - 50°C was continued for 1 hour and overnight (16 hours) without heating. Completion of the reaction was confirmed by NMR. The reaction mixture was poured into a well stirred ice- water mixture (ca. 38 L) in a 50-L reactor. The resulting suspension was stirred for 2 hours. The aqueous phase was separated from the sticky solid on the bottom of the reactor and discarded after neutralization to pH 5. The sticky residue was washed with water (6 L) and then dissolved in ethyl acetate (32 L). The resulting organic solution was washed with 20% aqueous NaOH solution (3.2 L). The formed solid was separated by filtration, washed with ethyl acetate and discarded. The aqueous phase was separated from the organic filtrate and discarded. The combined ethyl acetate extract was washed with 10% NaHCO3 solution (3 L), brine (5 L), then dried over MgSO4 (1.5 kg). The solid of MgSO4 hydrate was separated by filtration. The filtrate was dried over molecular sieves (1 kg), filtered, and evaporated in vacuo to an orange solid. The solid was washed with methyl t-butyl ether (2 L) and dried in vacuo at 40 °C over phosphorus pentoxide to constant weight to give the product in a yield of 1783.2 g (5.385 mol, 85%). HPLC purity of this material was determined to be 97%. 1H NMR (300 MHz, methanol-d4) 3.71-3.76 (2H, m, CH2-Br), 4.37-4.42 (2H, m, CH2-0), 6.86-6.90 (2H, m, Ar), 7.02-7.06 (2H, m, Ar), 7.65-7.75 (4H, m, Ar); 13C NMR (75 MHz, methanol-d4) 30.4, 69.6, 115.3, 116.1, 130.3, 132.4, 133.3, 133.7, 163.1, 163.3, 196.7.
References:
WO2017/70651,2017,A1 Location in patent:Paragraph 0062-0063
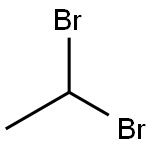
611-99-4
449 suppliers
$6.00/5g

106-93-4
400 suppliers
$15.00/5g

79578-62-4
10 suppliers
$1320.00/1g
557-91-5
65 suppliers
$45.00/100mg

79578-62-4
10 suppliers
$1320.00/1g
![Methanone, [4-(2-bromoethoxy)phenyl][4-[(tetrahydro-2H-pyran-2-yl)oxy]phenyl]-](/CAS/20210305/GIF/185223-77-2.gif)
185223-77-2
0 suppliers
inquiry

79578-62-4
10 suppliers
$1320.00/1g
36603-49-3
109 suppliers
$13.00/5g

79578-62-4
10 suppliers
$1320.00/1g