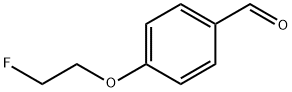
4-(2-FLUOROETHOXY)-BENZALDEHYDE synthesis
- Product Name:4-(2-FLUOROETHOXY)-BENZALDEHYDE
- CAS Number:2967-92-2
- Molecular formula:C9H9FO2
- Molecular Weight:168.16
Yield:2967-92-2 100%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 40; for 16 h;Inert atmosphere;
Steps:
4-(2-Fluoroethoxy)benzaldehyde (46)
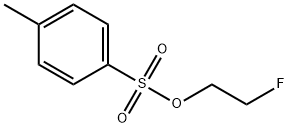
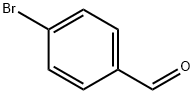
To a suspension of 4-hydroxybenzaldehyde 45 (0.98 g, 8.0 mmol, 1.0 equiv.) and potassium carbonate (1.66 g, 12.0 mmol, 1.5 equiv.) in N,N-dimethylformamide (6 mL) at RT was added a solution of 2-fluoroethyl 4-methylbenzenesulfonate (3.48 g, 16.0 mmol, 2 equiv.) in N,N-dimethylformamide (2 mL) followed by stirring at 40 °C for 16 h. Upon cooling to ambient temperature the reaction mixture was partitioned between water (100 mL) and diethyl ether (100 mL), the aqueous layer was isolated and further extracted with diethyl ether (2 × 100 mL). The combined organic fractions were washed with aqueous sodium hydroxide (1 M, 100 mL), brine, dried (Na2SO4) and concentrated under reduced pressure to give a crude residue. Purification by gradient column chromatography on silica gel (65:35 60:40 v/v hexanes:Et2O) to afford the title compound 46 as an opaque oil (1.34 g, quantitative); Rf 0.27 (60:40 v/v hexanes:Et2O); IR (ZnSe) 2962, 1687 (C=O), 1601, 1509, 1256, 1162, 1073, 833, 731, 518; 1H NMR (500 MHz, CDCl3) δ 9.90 (1H, s, CHO), 7.84 (2H, d, J = 8.3 Hz, ArH), 7.04 (2H, d, J = 8.3 Hz, ArH), 4.84-4.75 (2H, dt, 2JH-F = 47 Hz, JH-H = 4.0 Hz, CH2F), 4.33-4.28 (2H, dt, 3JH-F = 28 Hz, JH-H = 4.0 Hz, OCH2); 13C NMR (125.8 MHz, CDCl3) δ 190.9 (CHO), 163.5 (C), 132.2 (aryl), 129.5 (C), 115.0 (aryl), 81.7 (d, 1JC-F = 176 Hz, CH2F), 67.4 (d, 2JC-F = 25 Hz, OCH2); m/z (GC-MS) 161.10 ([M]+, C9H9FO2, 60%), 121.06 ([M-(CH2)2F]+, 100%), 93.01 ([C7H8]+, 30%), 65.08 ([C5H5]+, 26%).
References:
Moussa, Iman A.;Banister, Samuel D.;Manoli, Miral;Doddareddy, Munikumar Reddy;Cui, Jinquan;MacH, Robert H.;Kassiou, Michael [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 17,p. 5493 - 5497] Location in patent:supporting information; experimental part
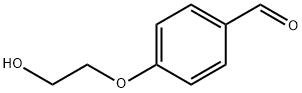
22042-73-5
138 suppliers
$6.00/250mg

2967-92-2
8 suppliers
inquiry