
4-(2-FLUOROPHENOXY)ANILINE synthesis
- Product Name:4-(2-FLUOROPHENOXY)ANILINE
- CAS Number:305801-12-1
- Molecular formula:C12H10FNO
- Molecular Weight:203.21

1478-03-1
7 suppliers
inquiry

305801-12-1
31 suppliers
$52.00/500mg
Yield:305801-12-1 96%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 70; for 3.5 h;
Steps:
4-(2-fluorophenoxy)benzenamine (4)
To the mixture of l-fluoro-2-(4-nitrophenoxy)benzene 3 (16.2 g, 69.3 mmol), saturated aqueous NH4C1 solution (30 mL) and EtOH (150 mL) was added iron powder (19.4 g, 347 mmol) slowly and then the resulting mixture was heated to 70 °C for 3.5 h. After being cooled to rt, the mixture was filtered. The filtrate was concentrated in vacuo. The residue was dissolved in EtOAc (160 mL) and washed successively with water (100 mL x 2) and brine (100 mL x 2). The organic phase was dried over Na2S04, filtered and concentrated in vacuo to afford the title compound (13.6 g, 96%) as yellow solid. 1H NMR (400 MHz, DMSO- d6) δ 7.35-7.28 (m, 1H), 7.14-7.00 (m, 2H), 6.93-6.85 (m, 1H), 6.80-6.73 (m, 2H), 6.64-6.56 (m, 2H), 4.99 (brs, 2H).
References:
WO2015/48662,2015,A2 Location in patent:Page/Page column 86
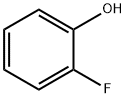
367-12-4
421 suppliers
$5.00/10g

305801-12-1
31 suppliers
$52.00/500mg
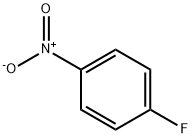
350-46-9
514 suppliers
$10.60/1gm:

305801-12-1
31 suppliers
$52.00/500mg