
4-(2-HYDROXY-ETHYL)-CYCLOHEXANONE synthesis
- Product Name:4-(2-HYDROXY-ETHYL)-CYCLOHEXANONE
- CAS Number:32863-01-7
- Molecular formula:C8H14O2
- Molecular Weight:142.2
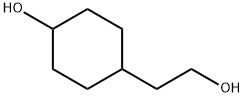
74058-21-2
42 suppliers
$131.00/1g

32863-01-7
24 suppliers
$155.00/50mg
Yield:32863-01-7 95%
Reaction Conditions:
Stage #1: 4-(2-Hydroxyethyl)cyclohexanolwith acetic acid at 18 - 21; for 0.583333 h;
Stage #2: with sodium hypochlorite in water; for 0.75 h;
Steps:
U.2
To a solution of 10 g (69 mmol) of 4-(2-hydroxyethyl)cyclohexanol in 50 mL of acetic acid maintained at a temperature between 18 and 210C are added over 35 min 35.9ml_ (79mmol) of an aqueous sodium hypochlorite solution. The mixture is further stirred for 45 min, TLC analysis indicating disappearance of starting material. Isopropanol (0.8 mL) is added, followed 10 min later by water (75 mL) and dichloromethane (100 mL). The two phases are separated by decantation and the
References:
WO2007/148208,2007,A2 Location in patent:Page/Page column 92-93
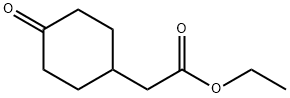
58012-34-3
110 suppliers
$12.00/250mg

32863-01-7
24 suppliers
$155.00/50mg
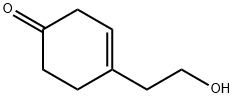
90199-14-7
0 suppliers
inquiry

32863-01-7
24 suppliers
$155.00/50mg
![2-(1,4-DIOXA-SPIRO[4.5]DEC-8-YL)-ETHANOL](/StructureFile/ChemBookStructure9/GIF/CB4146708.gif)
135761-76-1
27 suppliers
$65.00/10mg

32863-01-7
24 suppliers
$155.00/50mg
![ethyl 2-(1,4-dioxaspiro[4.5]decan-8-ylidene)acetate](/CAS/GIF/51656-91-8.gif)
51656-91-8
72 suppliers
$37.00/100mg

32863-01-7
24 suppliers
$155.00/50mg