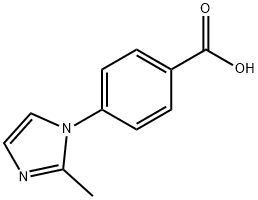
4-(2-methyl-1H-imidazol-1-yl)-Benzoic acid synthesis
- Product Name:4-(2-methyl-1H-imidazol-1-yl)-Benzoic acid
- CAS Number:101184-11-6
- Molecular formula:C11H10N2O2
- Molecular Weight:202.21
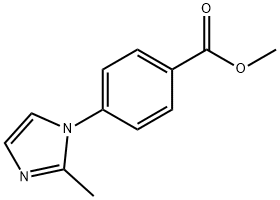
900015-35-2
13 suppliers
inquiry

101184-11-6
27 suppliers
inquiry
Yield:101184-11-6 93%
Reaction Conditions:
Stage #1: 4-(2-methyl-imidazol-1-yl)-benzoic acid methyl esterwith water;sodium hydroxide in methanol at 20; for 20 h;
Stage #2: with hydrogenchloride in methanol;water at -15; pH=1 - 2; for 0.0833333 h;
Steps:
Synthesis of 4-(2-methyl-imidazol-l-yl)-benzoic acid; To a stirring solution of 4-(2-methyl-imidazol-l-yl)-benzoic acid methyl ester (250 mg) in MeOH (2 mL) add 10 M NaOH aqueous solution (2.3 mL) and stir the reaction at rt for 20 h. Dilute with water (25 mL) and cool to -15°C. Quench with careful dropwise addition of cone. HC1 (2.2 mL) over 5 min to a final pH of 1-2. Wash the suspension with EtOAc (50 mL). Concentrate the aqueous layer to a solid, and further dry in vacuo under P2O5 to remove all moisture. Triturate the solid in MeOH and filter to remove inorganic salts, washing with MeOH. Concentrate the filtrate, triturate the residue in EtOAc/hexane, and filter, washing with hexane. Suspend the solid in 10% MeOH/EtOAc (20 mL) and stir/sonicate. Syringe filter the suspension to remove inorganics solids, concentrate the organic to a solid residue, and repeat this procedure. Triturate the organic residue in EtOAc/hexane and filter/dry the solid to afford the desired product 4-(2-methyl-imidazol- l-yl)-benzoic acid as a solid in 93% yield (238 mg, 1.06 mmol).
References:
WO2012/6203,2012,A1 Location in patent:Page/Page column 64
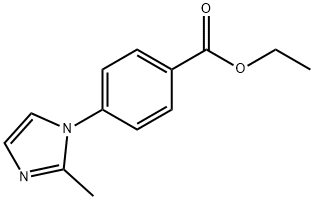
108035-44-5
5 suppliers
inquiry

101184-11-6
27 suppliers
inquiry
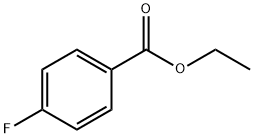
451-46-7
285 suppliers
$5.00/5g

101184-11-6
27 suppliers
inquiry