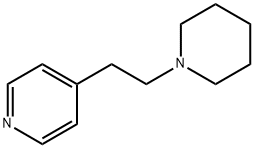
4-(2-PIPERIDINOETHYL) PYRIDINE synthesis
- Product Name:4-(2-PIPERIDINOETHYL) PYRIDINE
- CAS Number:13450-67-4
- Molecular formula:C12H18N2
- Molecular Weight:190.28

110-89-4
3 suppliers
$15.96/100ML
100-43-6
152 suppliers
$22.19/5ml

13450-67-4
10 suppliers
$33.39/250mg
Yield: 95%
Reaction Conditions:
with DE-PrIm-HPW (6b) in acetonitrile at 50; for 2 h;Michael Addition;Reagent/catalyst;
Steps:
Aza-Michael-type addition of amines to vinyl pyridine by catalysts 6a and 6b
General procedure: In a 10 mL flask, 2-vinyl pyridine (0.53 g, 0.54 mL, 5 mmol, 1 equiv.) and aniline (0.47 g, 0.46 mL, 5 mmol, 1 equiv.) were added to a mixture of catalyst 6b (0.1 g) in acetonitrile (5 mL) and heated for 2 h at 50 °C (Scheme 2). The progress of the reaction was checked by TLC. After the completion of the reaction, the solid catalyst was filtered, and the solvent was evaporated. The residue was added to toluene : sodium hydroxide (33%) (1:1) biphasic system. Then the aqueous phase was extracted with dichloromethane (pH = 5.0) to remove impurities. The organic layer was evaporated, and the precipitate was washed with acetone and was dried to afford N-(2-(pyridin-2-yl)ethyl)aniline 7a as a light brown solid (1.43 g, 95%). As indicated in Table 3, this reaction was performed with different amines in thermal conditions. The reaction condition of 6a approach is similar to the procedure described above for the 6b approach. The compound 7a was synthesized as a light brown solid (1.25 g, 83%).
References:
Ghasemi, Mohammad Hadi;Kowsari, Elaheh [Research on Chemical Intermediates,2017,vol. 43,# 7,p. 3691 - 3709]