
4-(2-PYRIDINYLMETHOXY)BENZALDEHYDE synthesis
- Product Name:4-(2-PYRIDINYLMETHOXY)BENZALDEHYDE
- CAS Number:57748-41-1
- Molecular formula:C13H11NO2
- Molecular Weight:213.23
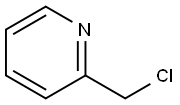
4377-33-7
103 suppliers
$90.00/250mg

123-08-0
928 suppliers
$5.00/10g

57748-41-1
25 suppliers
$45.00/50mg
Yield:57748-41-1 88%
Reaction Conditions:
Stage #1: 4-hydroxy-benzaldehydewith potassium carbonate in acetonitrile; for 0.5 h;
Stage #2: 2-chloromethylpyridine in acetonitrile; for 8 h;Reflux;
Steps:
4.2.7 General procedure for the synthesis of benzaldehydes 9a-13a, 9b-13b and 9c-13c
General procedure: The corresponding hydroxybenzaldehyde (1a-1c) was dissolved in dry acetonitrile (15-20mL) and K2CO3 (1.5 eq) was added. The reaction mixture was stirred for 30min and the appropriate halide (1 eq) was added and stirring was continued overnight at reflux temperature. The course of the reaction was monitored by TLC. After completion of the reaction, the solvent was evaporated under reduced pressure and the residue was purified by column chromatography.
References:
Kelly, John M.;Taylor, Martin C.;Baji?, Miroslav;Bokuli?, Ana;Jeli?, Dubravko;Ko?trun, Sanja;Krstulovi?, Luka;Popov, Andrea Bistrovi?;Rai?-Mali?, Silvana;Stojkovi?, Marijana Radi?;Zonji?, Iva [European Journal of Medicinal Chemistry,2020,vol. 207,art. no. 112802]
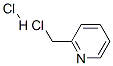
6959-47-3
362 suppliers
$7.00/10g

123-08-0
928 suppliers
$5.00/10g

57748-41-1
25 suppliers
$45.00/50mg
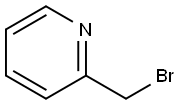
55401-97-3
53 suppliers
inquiry

123-08-0
928 suppliers
$5.00/10g

57748-41-1
25 suppliers
$45.00/50mg
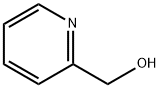
586-98-1
413 suppliers
$6.00/10g

123-08-0
928 suppliers
$5.00/10g

57748-41-1
25 suppliers
$45.00/50mg
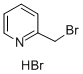
31106-82-8
183 suppliers
$10.00/1g

123-08-0
928 suppliers
$5.00/10g

57748-41-1
25 suppliers
$45.00/50mg