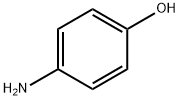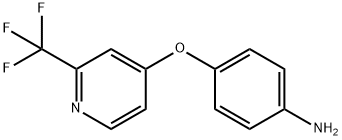
4-(2-Trifluoromethyl-pyridin-4-yloxy)-phenylamine synthesis
- Product Name:4-(2-Trifluoromethyl-pyridin-4-yloxy)-phenylamine
- CAS Number:630125-71-2
- Molecular formula:C12H9F3N2O
- Molecular Weight:254.21

850246-04-7
17 suppliers
inquiry

630125-71-2
6 suppliers
inquiry
Yield:630125-71-2 79%
Reaction Conditions:
Stage #1: 4-amino-phenolwith potassium tert-butylate in N,N-dimethyl acetamide at -5 - 20;
Stage #2: 4-fluoro-2-(trifluoromethyl)pyridine in N,N-dimethyl acetamide at 25; for 18 h;
Steps:
2U
Intermediate 2U: Preparation of 4-{[2-(trifluoromethyl) pyridin-4-yl] oxy} aniline; A cold (-5 °C), de-gassed solution of 4-aminophenol (41.6 g, 0.38 mol) in N, N- dimethylacetamide (250 mL) was treated with potassium tert-butoxide and stirred while warming to 20 °C. A solution containing 4-fluoro-2-trifluoromethylpyridine (60 g, 0.36 mol) in dimethylacetamide (150 mL) was slowly added and the mixture was stirred at 25 °C for 18 h. The reaction mixture was then concentrated in vacuo and the residue was added to vigorously stirred water (1 L). The precipitated solids were collected by suction filtration and washed with isopropanol/ether (1: 1) followed by ether and hexane. The yellow tan solids were dried to afford 72.8 g (79%) of product. 1H NMR (DMSO-d6) 8 5.20 (s, 2H,- NH2), 6.62 (m, 2H), 6.86 (m, 2H), 7.04 (dd, 1 H, J=5. 6,2. 4 Hz), 7.24 (d, 1H, J=2. 4 Hz), 8.54 (d, 1H, 5.7 Hz). MS ES 255 (M+H) +, calcd 255, RT=1.66 min.
References:
WO2005/35507,2005,A2 Location in patent:Page/Page column 65
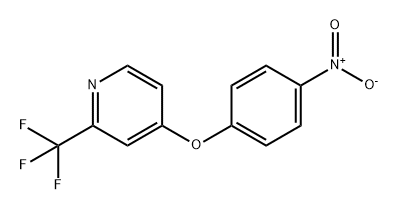
630125-72-3
1 suppliers
inquiry

630125-71-2
6 suppliers
inquiry