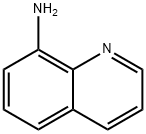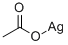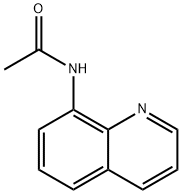
4-22-00-04712 (Beilstein Handbook Reference) synthesis
- Product Name:4-22-00-04712 (Beilstein Handbook Reference)
- CAS Number:33757-42-5
- Molecular formula:C11H10N2O
- Molecular Weight:186.21
Yield:33757-42-5 91%
Reaction Conditions:
with oxygen;3,6-di(2'-pyridyl)-1,2,4,5-tetrazine in tetrahydrofuran at 20; for 5 h;
Steps:
General experimental procedure for the synthesis of amide
General procedure: Thioacids (1 mmol, 1.0 equiv.), pytz (0.1 mmol, 0.1 equiv.) in THF (5 mL) were placed in a 50 mL round bottom flask and amines (1 mmol, 1.0 equiv.) dissolved in 5 mL THF was then added to it slowly under oxygen. The reaction mixture was stirred at room temperature for 4-7 h. When the reaction was completed (monitored by TLC), reaction mixture was evaporate to dryness at room temperature under vacuum. The residue was re-dissolved in chloroform (5 mL), washed with water and brine and dried over anhydrous Na2SO4. After filtration and removal of solvent, the residue was purified by flash column chromatography (hexane/ethyl acetate, 10:1) to afford the desired products 3aa-3as, 3ba-3bc, 3bt.
References:
Samanta, Suvendu;Ray, Shounak;Bhaduri, Samanka Narayan;Samanta, Partha Kumar;Biswas, Papu [Tetrahedron Letters,2020,vol. 61,# 35,art. no. 152272] Location in patent:supporting information