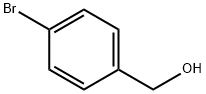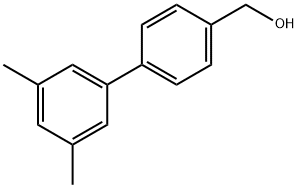
4-(3,5-Dimethylphenyl)benzyl alcohol synthesis
- Product Name:4-(3,5-Dimethylphenyl)benzyl alcohol
- CAS Number:885963-96-2
- Molecular formula:C15H16O
- Molecular Weight:212.29
Yield:885963-96-2 98 %Spectr.
Reaction Conditions:
with C47H62N4O4PdS2;potassium carbonate in water at 60; for 0.166667 h;Green chemistry;Suzuki-Miyaura Coupling;
Steps:
Catalysis Experiments
General procedure: In a typical in situ catalyst generation approach for the C-C coupling reaction, (4-bromophenyl)methanol (0.19 g, 1.0 mmol), phenylboronic acid (0.46 g, 1.2 mmol), K2CO3 (0.14 g, 1.0 mmol), Pd(OAc)2 (0.2 mol-% relative to the aryl bromide), and a given ligand (0.4 mol-%) were weighed into a 10 mL round-bottom flask equipped with a magnetic stirrer and reacted in water (4 mL) for a given reaction duration. In the case of a preformed palladium(II) complex, 0.2 mol-% equivalent relative to the aryl bromide was used in place of the ‘Pd(OAc)2+ligand’ deployed for the in situ complex generation. After the reaction, an aliquot of the reaction mixture was transferred into a clean conical flask and the solvent was removed under vacuum. The residue was collected in deuterated DMSO and its 1H NMR spectrum analysed. The yields were evaluated by comparing integration values for the NMR signal for the methylene CH2 protons of (4-bromophenyl)methanol at ~4.4 ppm against the CH2 function of the resulting biphenyl products at ~4.7 ppm (see Fig. S1 in the Supplementary Material for illustration).[59,60] The biphenyl products have been characterised in our previous studies.[19,59,61]
References:
Oloyede, Hammed Olawale;Akong Akong, Raymond;Woods, Joseph Anthony Orighomisan;G?rls, Helmar;Plass, Winfried;Eseola, Abiodun Omokehinde [Australian Journal of Chemistry,2021,vol. 74,# 2,p. 101 - 110]