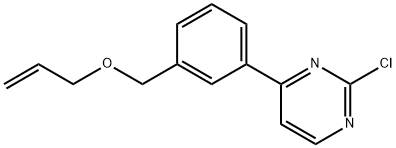
4-(3-(allyloxymethyl)phenyl)-2-chloropyrimidine synthesis
- Product Name:4-(3-(allyloxymethyl)phenyl)-2-chloropyrimidine
- CAS Number:937273-29-5
- Molecular formula:C14H13ClN2O
- Molecular Weight:260.72
Yield:937273-29-5 84.7 %
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium hydroxide at 20;
Steps:
1.2 (2) 4-(3-(allyloxymethyl)phenyl)-2-chloropyrimidine (V-1)
In reaction bottle, add compound III-1 (5.0g, 22.7mmol),3-bromopropene (IV-1, 25 mL), potassium hydroxide (2.5 g, 45.4 mmol), tetrabutylammonium iodide (0.4 g, 1.1 mmol), and stirred at room temperature overnight. The reaction system was detected by TLC spot plate (PE/EA=2:1), and the raw materials disappeared under the ultraviolet analyzer at 254nm, and the product no longer increased, and the reaction was judged to be terminated. Added 30 mL of water, stirred for 10 minutes, allowed to stand and separated layers, and collected the organic phase. The aqueous phase was extracted with 20 mL of dichloromethane × 2, the organic phases were combined, and the aqueous phase was discarded. The organic phase was washed with 20 mL of saturated brine, and after standing for separation, the aqueous phase was discarded, and the organic phase was dried with an appropriate amount of anhydrous sodium sulfate. After drying, the desiccant was filtered off, and the filtrate was concentrated to dryness under reduced pressure to obtain a residue. Pass the residue through a flash column (the elution system is ethyl acetate/petroleum ether, the ratio gradually increases from 1:20 to 1:5), collect the eluate of the corresponding product, and concentrate the eluent to dryness under reduced pressure to obtain Compound V-1 (5 g, 84.7%).
References:
CN115490705,2022,A Location in patent:Paragraph 0106; 0111-0113
87199-15-3
294 suppliers
$8.00/5g

937273-29-5
28 suppliers
inquiry
![[3-(2-Chloro-pyrimidin-4-yl)-phenyl]-methanol](/StructureFile/ChemBookStructure23/GIF/CB81076271.gif)
