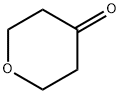
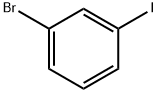
4-(3-BROMOPHENYL)-TETRAHYDRO-2H-PYRAN-4-OL synthesis
- Product Name:4-(3-BROMOPHENYL)-TETRAHYDRO-2H-PYRAN-4-OL
- CAS Number:135048-94-1
- Molecular formula:C11H13BrO2
- Molecular Weight:257.12
Yield:135048-94-1 85.1%
Reaction Conditions:
Stage #1: 1,3-dibromobenzenewith n-butyllithium in tetrahydrofuran;ethanol at -72; for 0.5 h;Inert atmosphere;
Stage #2: tetrahydropyran-4-one in tetrahydrofuran;ethanol at 20;
Steps:
1.1 (1) Synthesis of Intermediate 11
1,3-Dibromotoluene (42.4mmol, 10g) was dissolved in 170mL of THF, the reaction system was evacuated and replaced with nitrogen three times, the reaction flask was placed in a dry ice-ethanol system to cool down to -72°C, and n- BuLi (2.5M, 48.7 mmol, 19.5 mL) was added dropwise and reacted for 30 min, then tetrahydropyranone (50.88 mmol, 5.1 g) was added dropwise to it, slowly returned to room temperature after the dropwise addition, and reacted overnight. The reaction was complete as detected by TLC. Water was added to quench the reaction, the THF layer was separated, the aqueous phase was extracted three times with EA, the organic phases were combined, dried, rotary evaporated, and purified by column chromatography to obtain the product intermediate 11 (9.24 g, 36.1 mmol, yield 85.1%).
References:
CN113735911,2021,A Location in patent:Paragraph 0232-0238