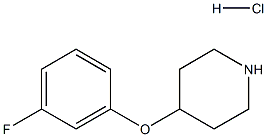
4-(3-fluorophenoxy)piperidine(HCl) synthesis
- Product Name:4-(3-fluorophenoxy)piperidine(HCl)
- CAS Number:3202-36-6
- Molecular formula:C11H15ClFNO
- Molecular Weight:231.7

3202-35-5
26 suppliers
$121.00/250mg

3202-36-6
34 suppliers
$57.00/250mg
Yield:-
Reaction Conditions:
with hydrogenchloride in methanol;diethyl ether;
Steps:
Description 14 :4-[(3-Fluorophenyl)oxy]piperidine (D14). A solution of D13 (16.4g, 55mmol) in DCM (200ml) at 0°C was treated drop-wise with TFA (17ml). The reaction was warmed to room temperature for 2.5 hrs and left overnight. The solvent was then removed in vacuo and the residue partitioned between DCM and 2M NaOH solution. The aqueous was further extracted with DCM (x2) and the combined organics concentrated in vacuo. The residue was redissolved in DCM and extracted with 2M HCI (x2) which was then basified and re-extracted with DCM. The combined organics were concentrated in vacuo to give the title compound (12g). δH (CDCI3, 250MHz) 1.66 (2H, m), 2.01 (2H, m), 2.73 (2H, m), 3.14 (2H, m), 4.34 (1 H, m), 6.68 (3H, m), 7.19 (1 H, m), MS (ES): MH+ 196. This whole was diluted with MeOH and treated with 1 M HCI in Et2O to give the hydrochloride salt of the title compound (8.Og).
References:
WO2007/12479,2007,A2 Location in patent:Page/Page column 35-36
920511-29-1
3 suppliers
inquiry

3202-36-6
34 suppliers
$57.00/250mg
372-20-3
366 suppliers
$7.00/5g

3202-36-6
34 suppliers
$57.00/250mg

109384-19-2
470 suppliers
$5.00/1g

3202-36-6
34 suppliers
$57.00/250mg