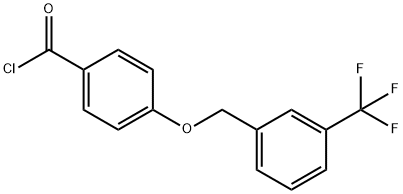
4-{[3-(trifluoromethyl)benzyl]oxy}benzoyl chloride synthesis
- Product Name:4-{[3-(trifluoromethyl)benzyl]oxy}benzoyl chloride
- CAS Number:632366-17-7
- Molecular formula:C15H10ClF3O2
- Molecular Weight:314.69
![4-{[3-(trifluoromethyl)benzyl]oxy}benzoic acid](/CAS/GIF/632366-16-6.gif)
632366-16-6
8 suppliers
$126.00/500mg
![4-{[3-(trifluoromethyl)benzyl]oxy}benzoyl chloride](/CAS/GIF/632366-17-7.gif)
632366-17-7
6 suppliers
inquiry
Yield:-
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane;
Steps:
38 Example 38: Synthesis [OF 6-CYCLOHEXYL-7-OXO-5- [4- (3-TRIFLUOROMETHYL-BENZYLOXY)-PHENYL]-] 4, [7-DIHYDRO-PYRAZOLO] [1, 5-a] pyrimidine-2-carboxylic acid
To a solution of 2.28 g (15 mmol) [OF 4-LAYDROXY-BENZOIC ACID NAETHYL ESTER] in 30 mL of [DIMETHYLFORMAMIDE] (DMF) was added 5.38 g (16.5 mmol) of cesium carbonate (Cs2CO3), followed by 2.75 mL (18 mmol) of [3-(TRIFLUOROMETHYL)-BENZYL BROMIDE,] and the resulting heterogeneous mixture was stirred at rt overnight. The reaction mixture was filtered and concentrated, diluted with water and extracted with ethyl acetate, and the combined organic extracts were washed with water and brine, and concentrated to give 4.75 g of desired [4- (3-] [TRIFLUOROMETHYL-BENZYLOXY)-BE71ZOIC ACID METHYL ESTER] as indicated [BY H] NMR [AND 19F-NMR] (containing traces of residual 3- (trifluoromethyl)-benzyl bromide). This product was used without any further purification in the synthesis of 6-cyclohexyl- 7-oxo-5-[4-(3-trifluoromethyl-benzyloxy)-phenyl]-4,7-dihydro-pyrazolo[1,5-a]pyrimidine-2- carboxylic acid (545), which was accomplished as depicted in the above scheme, via standard transformations that were previously described for the synthesis of 5- (4-Benzyloxy-phenyl)-6- cyclohexyl-7-oxo-4,7-dihydro-pyrazolo [[1,] 5-a] pyrimidine-2-carboxylic acid.
References:
WO2003/101993,2003,A1 Location in patent:Page 73-74
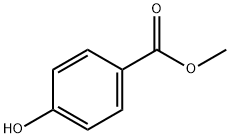
99-76-3
749 suppliers
$5.00/25g
![4-{[3-(trifluoromethyl)benzyl]oxy}benzoyl chloride](/CAS/GIF/632366-17-7.gif)
632366-17-7
6 suppliers
inquiry
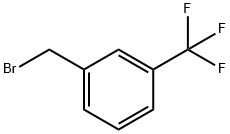
402-23-3
255 suppliers
$6.00/1g
![4-{[3-(trifluoromethyl)benzyl]oxy}benzoyl chloride](/CAS/GIF/632366-17-7.gif)
632366-17-7
6 suppliers
inquiry
![Benzoic acid, 4-[[3-(trifluoromethyl)phenyl]methoxy]-, methyl ester](/CAS/20210305/GIF/632366-15-5.gif)
632366-15-5
1 suppliers
inquiry
![4-{[3-(trifluoromethyl)benzyl]oxy}benzoyl chloride](/CAS/GIF/632366-17-7.gif)
632366-17-7
6 suppliers
inquiry