4(3H)-Pyrimidinone, 2-amino-6-hydroxy-5-propyl- synthesis
- Product Name:4(3H)-Pyrimidinone, 2-amino-6-hydroxy-5-propyl-
- CAS Number:500161-22-8
- Molecular formula:C7H11N3O2
- Molecular Weight:169.18
Yield:500161-22-8 94%
Reaction Conditions:
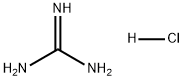
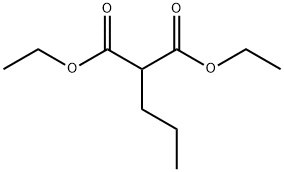
Stage #1: guanidinium monohydrochloride;diethyl 2-propylpropanedioatewith natrium in ethanol at 20; for 4 h;Inert atmosphere;
Stage #2: in ethanol; for 1 h;Inert atmosphere;Reflux;
Steps:
General procedure for the preparation of 5-substituted2-amino-4,6-dihydroxypyrimidines A2-A11
General procedure: Metallic sodium (12.9 g, 0.56 mol) was dissolved in absolute ethanol (300 mL) under argon while being intensively stirred with a mechanical stirrer. The reaction flask was equipped with a reflux condenser with a chlorocalcium tube. After all the sodium was dissolved and the reaction mixture was cooled to room temperature; guanidine hydrochloride(21.02 g, 0.22 mol) was added under intensive stirring, followed by the corresponding monosubstituted malonic acid diester (0.2 mol). The reaction mixture was further intensively stirred due to the production of the solid product,which is so massive that after 2 h it already practically precludes stirring. After another 2 h, absolute ethanol (200 mL) was added and the reaction mixture was refluxed for 1 h while being stirred. Afterward, ethanol (ca200-300 mL) was evaporated on a vacuum rotary evaporatorand water (500 mL) was added to the reaction mixture. After stirring, the product (in the form of sodium salt) was almost dissolved. The obtained mixture was subsequently neutralized by dropwise addition of acetic acid, resulting in immediate and quantitative precipitation of the desired product in the form of a fine solid. This mixture was subsequently heated under reflux for 10 min and then cooled to laboratory temperature. This heating and cooling was repeated twice to get a well-filterable solid product. The solid product was filtered off, washed with water (2 x 50 mL),ethanol (2 x 50 mL), and acetone (2 x 50 mL). The product was dried under high vacuum at 60 °C for 2 days. The obtained purity of the product prepared in this manner is sufficient for the following reaction and based on analyses contains only crystalline water.
References:
Jansa, Petr;Hol, Antonn;Dransk, Martin;Kolman, Viktor;Janeba, Zlatko;Kosteck, Petra;Kmonkov, Eva;Zdek, Zdenk [Medicinal Chemistry Research,2014,vol. 23,# 10,p. 4482 - 4490]