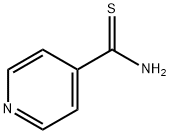
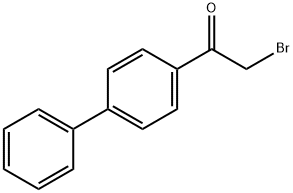
4-(4-Biphenylyl)-2-(4-pyridyl)thiazole, 97% synthesis
- Product Name:4-(4-Biphenylyl)-2-(4-pyridyl)thiazole, 97%
- CAS Number:102554-97-2
- Molecular formula:C20H14N2S
- Molecular Weight:314.4
Yield:102554-97-2 69%
Reaction Conditions:
in ethanol; for 18 h;Reflux;Inert atmosphere;
Steps:
General procedure for synthesis of aryl substituted 2-(pyridin-2-yl)thiazole 3a-e and 2-(pyridin-4-yl)thiazole 4a-e compounds:
General procedure: A solution 1 or 2 (1.2 mmol) of in dry EtOH (25 mL) was stirredunder N2 and refluxed. Then, the appropriate bromo substituted aromaticketone (1.5 mmol) in (15 mL) dry EtOH was added directly. Aftercooling to room temperature, the solvent was poured into water, andthe insoluble thiazole derivatives were allowed to settle. The residualsolid was washed by decantation with water. The solid was solved ethylacetate, evaporated, and the residue purified by column chromotography
References:
Ery?lmaz, Serpil;Türk ?eliko?lu, Emine;?dil, ?nder;?nkaya, Ersin;Kozak, Zehra;M?s?r, Ender;Gül, Melek [Bioorganic Chemistry,2020,vol. 95,art. no. 103476]