
4-(4-BROMO-BUTOXY)-BENZALDEHYDE synthesis
- Product Name:4-(4-BROMO-BUTOXY)-BENZALDEHYDE
- CAS Number:72621-19-3
- Molecular formula:C11H13BrO2
- Molecular Weight:257.12
Yield:72621-19-3 90%
Reaction Conditions:
with potassium carbonate in acetone; for 48 h;Reflux;
Steps:
t-Butyl-4-[4-(naphthalimide-2-yl)butyl]-1-piperazinecarboxylate (15a)
General procedure: Compound 2-(4-bromobutyl)naphthalimide (12b, 332mg, 1.2 mmol) in acetone (20 ml) was treated with anhydrous K2CO3 (552mg, 4 mmol) and N-Boc-piperazine (186 mg, 1 mmol) and the mixture was refluxed for 48h, until TLC indicated a complete consumption of starting material. Excess potassium carbonate was removed by filtration and the solvent was evaporated under vacuum. The crude product was purified by silica gel column chromatography using MeOH-CHCl3(0.5: 9.5) as an eluent to afford pure compound 15a as a light brown solid. Yield: 327 mg, 75%
References:
Kamal, Ahmed;Kumar, Pogula Praveen;Khan, Mohammed Naseer Ahmed;Sheshadri, Bobburi Naga;Srinivas, Olepu [Letters in drug design and discovery,2015,vol. 12,# 5,p. 374 - 384]

110-52-1
554 suppliers
$9.00/1g

123-08-0
893 suppliers
$5.00/10g
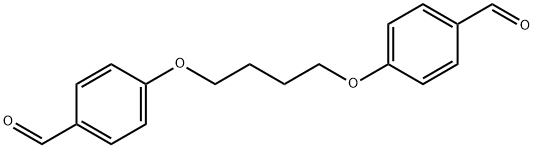
77355-00-1
3 suppliers
inquiry

72621-19-3
6 suppliers
inquiry