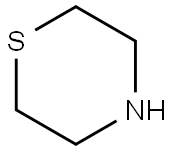
4-(4-BROMOBENZYL)THIOMORPHOLINE synthesis
- Product Name:4-(4-BROMOBENZYL)THIOMORPHOLINE
- CAS Number:17494-28-9
- Molecular formula:C11H14BrNS
- Molecular Weight:272.2
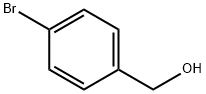
123-90-0
239 suppliers
$11.00/5g
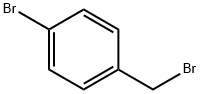
589-15-1
440 suppliers
$6.00/10g

17494-28-9
13 suppliers
$437.00/1g
Yield:17494-28-9 100%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 16 h;
Steps:
A mixture of 4-bromobenzyl bromide (1.0 g, 4.0 mmol), triethylamine (0.84 mL,6.0 mmol) and thiomorpholine (0.82 mL, 8.0 mmol) in THF (10 mL) was stirred at ambient temperature for 16 h. The solvent was evaporated and the resultant residue diluted with saturated aqueous sodium bicarbonate solution and extracted with ethyl acetate (2 x 50 mL). The combined organic layer was dried over sodium sulfate, filtered and evaporated to afford the title compound as an off-white solid (1.13 g, quantitative yield). 1H NMR (CDCl3, 300MHz): 7.46- 7.40 (m, 2H), 7.21-7.16 (m, 2H), 3.45 (s, 2H), 3.15-3.09 (m, 1H), 2.76-2.60 (m, 6H), 2.62-2.57 (m, 1H).
References:
WO2009/151598,2009,A1 Location in patent:Page/Page column 150