4'-(4-BROMOBENZYLOXY)ACETOPHENONE synthesis
- Product Name:4'-(4-BROMOBENZYLOXY)ACETOPHENONE
- CAS Number:694443-80-6
- Molecular formula:C15H13BrO2
- Molecular Weight:305.17
Yield:694443-80-6 96%
Reaction Conditions:
with potassium carbonate in acetone; for 3 h;Reflux;Inert atmosphere;
Steps:
5 Preparation of 1-(4-((4-bromobenzyl)oxy)phenyl)-3-(4-(tert-butyl) phenyl)propane-1 ,3-dione, 9
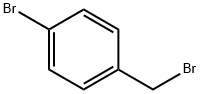
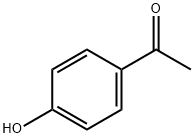
Example 5 - Preparation of 1-(4-((4-bromobenzyl)oxy)phenyl)-3-(4-(tert-butyl) phenyl)propane-1 ,3-dione, 9 A mixture of 4-hydroxyacetophenone (5.0 g, 36.7 mmol), 4- bromobenzyl bromide (13.78 g, 55.0 mmol) and potassium carbonate (10.15 g, 73.4 mmol) in acetone (185 mL) was heated at reflux under an atmosphere of nitrogen for 3 hours. The volume of acetone was reduced by rotary evaporation and the residue was partitioned between water and ethyl acetate. The organic phase was separated, washed with water followed by saturated sodium chloride solution and dried with magnesium sulfate. Evaporation in-vacuo gave the crude material as a colourless solid which was purified by column chromatography over silica gel eluting with 0-100% ethyl acetate: heptane. Evaporation of the eluents gave 1 -(4-((4-Bromobenzyl)oxy)phenyl)ethanone as a colourless solid (10.75 g, 96%). δΗ (CDCIs, 400 MHz) 7.92 (d, J 9.0, 2H), 7.50 (d, J 8.5, 2H), 7.28 (d, J8.5, 2H), 6.96 (d, J9.0, 2H), 5.06 (s, 2H), 2.53 (s, 3H). 5C (CDCI3, 100 MHz) 196.8, 162.4, 135.4, 132.0, 130.9, 130.8, 129.2, 122.3, 1 14.7, 69.5, 26.5. HRMS (ES): calc. for Ci5Hi4Br02 [MH+], 305.0172. Found, 305.0172 [MH+]. Calc. for Ci5Hi302BrNa [MNa+] 326.9991 . Found 326.9990 [MNa+].
References:
WO2015/6803,2015,A1 Location in patent:Paragraph 00138-00139