
4-(4-Bromophenyl)-2-thiazoleacetonitrile synthesis
- Product Name:4-(4-Bromophenyl)-2-thiazoleacetonitrile
- CAS Number:94833-31-5
- Molecular formula:C11H7BrN2S
- Molecular Weight:279.16
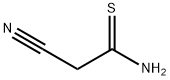
7357-70-2
167 suppliers
$15.00/1g
99-73-0
231 suppliers
$10.00/1g

94833-31-5
27 suppliers
$60.00/500mg
Yield:94833-31-5 57%
Reaction Conditions:
in ethanol at 50; for 4 h;Heating / reflux;
Steps:
5 Description 5. [4-(4'-bromo)phenylthiazol-2-yl]acetonitrile
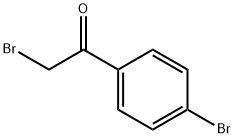
To a warm [(50°C)] solution of a-cyanothioacetamide (1.08 g, 10.8 mmol) in absolute EtOH (45 mL), [A-BROMO-4-BROMOACETOPHENONE] (3 g, 10.8 mmol) was added and the resultant mixture was refluxed for 4 hours. The reaction was complete as judged by TLC [(SI02] ; hexane/AcOEt 7: 3, Rf [No.0. ]7). After cooling, the mixture was stirred at room temperature overnight. Then the formed solid was filtered off, washed with [MEOH] and dried under vacuum (2 h, [50°C).] Pure [4- (4'-bromo) phenylthiazol-2-yl] acetonitrile (5,1. 73 g, 57%), as a brownish solid, was obtained. Analytical data ['H-NMR] [(DMSO-D6,] [6)] : 8.3 (s, 1H) ; 7.85 (d, 2H); 7.6 (d, 2H); 4.6 (s, 2H, [CH2-CN)]
References:
WO2003/105842,2003,A1 Location in patent:Page 66 - 67