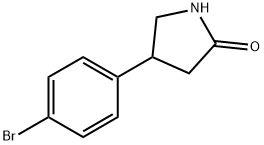
4-(4-Bromophenyl)pyrrolidine-2-one synthesis
- Product Name:4-(4-Bromophenyl)pyrrolidine-2-one
- CAS Number:28311-23-1
- Molecular formula:C10H10BrNO
- Molecular Weight:240.1
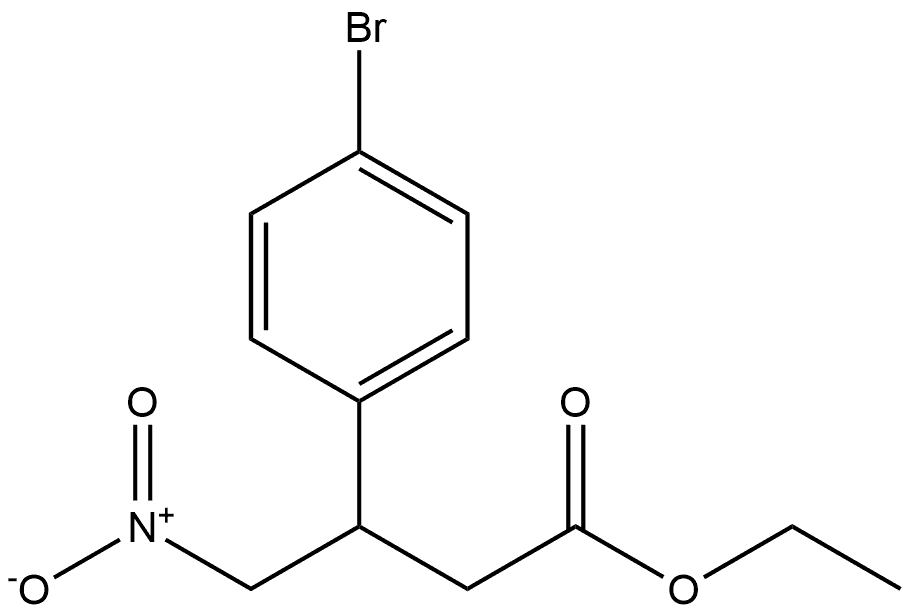
29655-72-9
0 suppliers
inquiry

28311-23-1
28 suppliers
$248.03/250mg
Yield:28311-23-1 61%
Reaction Conditions:
Stage #1: ethyl 3-(4-bromophenyl)-4-nitrobutanoatewith hydrogenchloride;ethanol;zinc in 1,4-dioxane at 0 - 20; for 1.66667 h;
Stage #2: with sodium ethanolate in ethanol at 20;
Steps:
5.1.186.A
B. 4-(4-Bromophenyl)pyrrolidin-2-one. Ethyl 3-(4-bromophenyl)-4- nitrobutanoate (2.02 g, 6.39 mmol) was dissolved in ethanol (80 mL) and 4 M hydrochloric acid in 1 ,4-dioxane (16 mL, 63.9 mmol) with stirring and cooled to 0 °C under nitrogen. Zinc dust (4.18 g, 63.9 mmol) was added slowly in portions over -10 min. Gas evolution was observed. After 30 min the cold bath was removed and the gray reaction mixture stirred 1 h at room temperature. The resulting mixture was filtered through Celite and the filter cake washed thoroughly with methanol. The filtrate was concentrated on a rotary evaporator. The residue was dissolved in ethanol (50 mL) with stirring at room temperature. Sodium ethoxide (24 mL of a 2.68 M solution in ethanol, 63.9 mmol) was added and the resulting cloudy mixture (pH is basic) stirred at room temperature overnight. The resulting cloudy mixture was poured into a separatory funnel containing dichloromethane (300 mL) and 1 M sodium bisulfate (80 mL). The mixture was shaken and the layers separated. The water layer was extracted once with dichloromethane. The combined organics were dried over magnesium sulfate, filtered, and concentrated on a rotary evaporator. The residue was dissolved in acetonitrile and water was added to induce precipitation of solids. The solids EPO
References:
WO2008/51493,2008,A2 Location in patent:Page/Page column 233-234
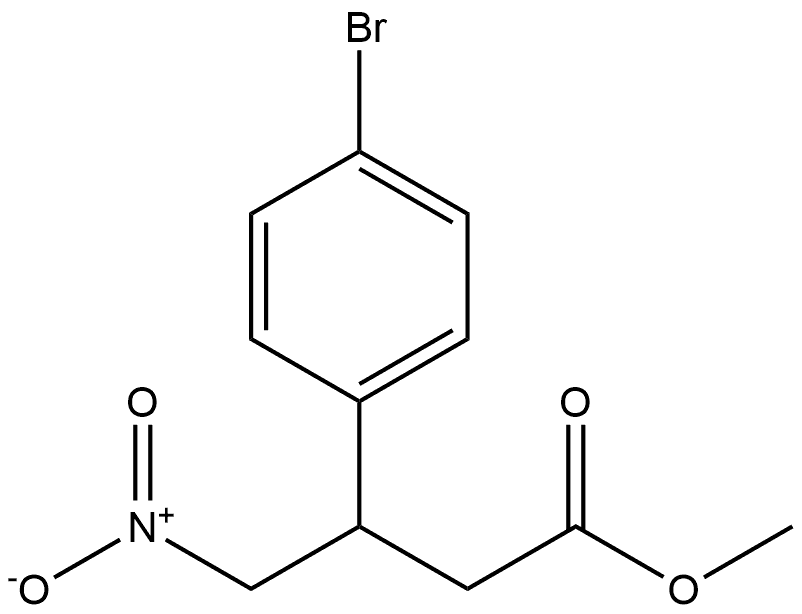
540481-48-9
0 suppliers
inquiry

28311-23-1
28 suppliers
$248.03/250mg

589-87-7
528 suppliers
$10.00/1g

28311-23-1
28 suppliers
$248.03/250mg
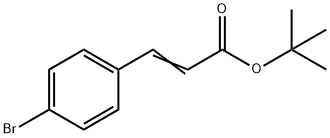
422519-72-0
0 suppliers
inquiry

28311-23-1
28 suppliers
$248.03/250mg