
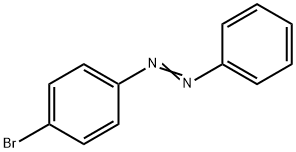
4-(4-broMophenylazo)phenol synthesis
- Product Name:4-(4-broMophenylazo)phenol
- CAS Number:3035-94-7
- Molecular formula:C12H9BrN2O
- Molecular Weight:277.12

106-40-1
458 suppliers
$12.00/5g

108-95-2
722 suppliers
$14.00/25g

3035-94-7
48 suppliers
$190.00/200mg
Yield: 98%
Reaction Conditions:
Stage #1:4-bromo-aniline with hydrogenchloride in water at 0 - 5; for 0.0833333 h;
Stage #2:phenol in water for 0.0833333 h;
Steps:
4.6. General Procedure for the Synthesis of Azo Dyes 4a-o
General procedure: Hydrochloric acid 37% (0.5) was poured on a vessel containing aniline derivatives which was located inside ice bath at temperature between 0-5oC. NFSP 10 was then added to the mixture while grinding to complete diazotization reaction. After forming diazonium salts of aniline derivatives, the phenolates compounds were added to the resulted vessel to produce desired azo dyes. Reaction times were listed according to different derivatives in the Table 2. After the end of reaction for separating catalyst from product, ethyl acetate was excessively added to the reaction mixture and mixture was filtered off. Azo dyes were obtained by removing the solvent of mixture. The products were characterized by their FT-IR, 1H-NMR and 13C-NMR spectra. In order to study there usability of the catalyst, the synthesis of 1-(4-nitrophenylazo)-2-naphthol was repeated in 5 times with the same catalyst. The obtained results are shown that the efficiency of the catalyst after the extract and reuse was appropriately close to each other (98±1.5%). 4.7.1. 4-(4-Bromophenylazo) Phenol (4a) Yield: 98%; mp: 171-173oC (lit.,27 168-170°C); FT-IR(KBr): 3388, 1632, 724, 512 cm-1; 1H NMR (300 MHz,CDCl3): δH 7.85 (dd, J = 9.1 Hz, 2H), 7.73 (dd, J = 9.1 Hz,2H), 7.61 (dd, J = 9.1 Hz, 2H), 6.93 (dd, J = 9.1 Hz, 2H),5.34 (s, OH); 13C NMR (75 MHz, CDCl3): δC 157.8 (C1),150.4 (C5), 145.7 (C4), 131.1 (C7), 124.9 (C6), 123.7 (C3),114.4 (C2), 94.5 (C8); Anal. Calc. for C12H9N2BrO: C,51.88; H, 3.35; N, 10.11%. Found: C, 51.84; H, 3.42; N,10.09%.
References:
Shomali, Ashkan;Valizadeh, Hassan;Noorshargh, Saeideh [Letters in Organic Chemistry,2017,vol. 14,# 6,p. 409 - 418]
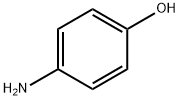
123-30-8
605 suppliers
$10.00/5g

106-40-1
458 suppliers
$12.00/5g

3035-94-7
48 suppliers
$190.00/200mg
4418-84-2
4 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3035-94-7
48 suppliers
$190.00/200mg