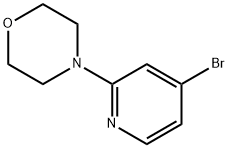
4-(4-Bromopyridin-2-yl)morpholine synthesis
- Product Name:4-(4-Bromopyridin-2-yl)morpholine
- CAS Number:1040377-12-5
- Molecular formula:C9H11BrN2O
- Molecular Weight:243.1

110-91-8
678 suppliers
$9.00/1g
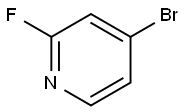
128071-98-7
284 suppliers
$7.00/1g

1040377-12-5
55 suppliers
$35.00/100mg
Yield: 90%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-d6-formamide at 20 - 100; for 12 h;Sealed tube;Solvent;Reagent/catalyst;Temperature;
Steps:
7.1; 24.4 Step 4. 4 -(4 -bromopyridin -2 -yl jmorpholine
4-Bromo-2-fluoropyridine (1.0 g, 5.68 mmol) was dissolved in 5 mL DMF and morpholine (0.60 mL, 6.82 mmol) and Cs2C03 (3.70 g, 11.36 mmol) were added at room temperature. The mixture was stirred in a sealed vial at 100 °C overnight. The mixture was portioned between H20 and EtOAc. The phases were separated, the aqueous layer was extracted with EtOAc (2x) and the combined organic layers washed with brine (lx), dried over anhydrous Na2S04 and evaporated to dryness. The crude material was purified via column chromatography on a 50 g silica gel column using as eluent a gradient of EtOAc in cyclohexane from 0 to 50%. The desired fractions were collected together to afford the title compound (1.24 g, 5.10 mmol, 90% yield) as a white solid. NMR (400 MHz, DMSO-d6) d 8.01 (d, J=5.3 Hz, 1H), 7.06 (d, J=l .3 Hz, 1H), 6.88 (dd, J=l .5, 5.3 Hz, 1H), 3.73 - 3.62 (m, 4H), 3.54 - 3.42 (m, 4H). MS-ESI (m/z) calcd for C9Hi2BrN20 [M+H]+: 243.01. Found 243.09/245.10.
References:
E-SCAPE BIO, INC.;GAROFALO, Albert W.;ANDREOTTI, Daniele;BERNARDI, Silvia;SERRA, Elena;MIGLIORE, Marco;SABBATINI, Fabio Maria;BEATO, Claudia;VINCETTI, Paolo;BUDASSI, Federica WO2019/222173, 2019, A1 Location in patent:Page/Page column 62-63; 85-87
35980-77-9
47 suppliers
$45.00/10mg

1040377-12-5
55 suppliers
$35.00/100mg