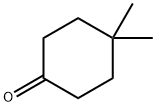
4 4-DIMETHYLCYCLOHEXANONE 97 synthesis
- Product Name:4 4-DIMETHYLCYCLOHEXANONE 97
- CAS Number:4255-62-3
- Molecular formula:C8H14O
- Molecular Weight:126.2
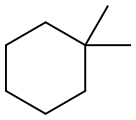
590-66-9
21 suppliers
$76.00/1g
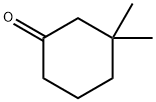
2979-19-3
227 suppliers
$6.00/1g
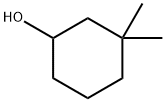
767-12-4
13 suppliers
inquiry
1193-47-1
93 suppliers
$87.00/250mg

4255-62-3
199 suppliers
$10.00/1g
Yield:4255-62-3 16 %Chromat. ,2979-19-3 35 %Chromat. ,767-12-4 17 %Chromat. ,1193-47-1 13 %Chromat.
Reaction Conditions:
with [(S,S)-Fe(1,1'-bis((5-(2,6-bis(trifluoromethyl)phenyl)pyridin-2-yl)methyl)-2,2'-bipyrrolidine)(acetonitrile)2](SbF6)2 ;dihydrogen peroxide;acetic acid in water;acetonitrile at 20; for 0.5 h;
Steps:
2.6.1 Reaction 6. Oxidation of 1,1-Dimethylcyclohexane
GC Yield Data. Run 1: 16% RSM, 16% S2, 35% S3a, 17% S3b, 13% S4, 5.3:1 distal:proximal. Run 2: 24% RSM, 14% S2, 28% S3a, 20% S3b, 11% S4, 5.8:1 distal:proximal. Run 3: 23% RSM, 13% S2, 26% S3a, 18% S3b, 10% S4, 5.6:1 distal:proximal. Average RSM: 21±4%. Average S2: 14±2%. Average S3a: 29±5%. Average S3b: 18±2%. Average S4: 11±2%. Average distal:proximal: 5.6±0.3:1. Data for (S,S)-Fe(PDP) (1) have been previously reported (Chen and White, Science 2010, 327, 566). (0224) (0225) 4,4-dimethylcyclohexanone (S2). 1,1-dimethylcyclohexane (S1) (33.7 mg, 0.3 mmol, 1.0 equiv, Sigma-Aldrich) was reacted with (S,S)-Fe(CF3-PDP) (2) according to Method A with nitrobenzene (60 mol %) added as an internal standard. Yields were determined by GC analysis of the crude reaction mixture after reaction completion. All product yields are calibrated for response factors relative to starting material, rounded to the nearest whole number and the average of three runs. 1H NMR (400 MHz, CDCl3) δ 2.34 (t, J=6.8 Hz, 4H), 1.67 (app t, J=7.2 Hz, 4H), 1.09 (s, 6H). (0226) (0227) 3,3-dimethylcyclohexanone (S3a). 1H NMR (400 MHz, CDCl3) δ 2.26 (app t, J=6.8 Hz, 2H), 2.15 (s, 2H), 1.91-1.84 (m, 2H), 1.59-1.57 (m, 2H), 0.97 (s, 6H). (0228) (0229) 3,3-dimethylcyclohexanol (S3b). 1H NMR (500 MHz, CDCl3) δ 3.78-3.68 (m, 1H), 1.98-1.91 (m, 1H), 1.71-1.57 (m, 2H), 1.49-1.37 (m, 1H), 1.32-1.24 (m, 2H), 1.13-1.01 (m, 2H), 0.95 (s, 3H), 0.89 (s, 3H). These spectral data match those reported in the literature (Kamata et al., Nature Chem. 2010, 2, 478). (0230) (0231) 2,2-dimethylcyclohexanone (S4). 1H NMR (400 MHz, CDCl3) δ 2.39 (app t, J=6.8 Hz, 2H), 1.86-1.80 (m, 2H), 1.77-1.70 (m, 2H), 1.68-1.64 (m, 2H), 1.11 (s, 6H).
References:
The Board of Trustees of the University of Illinois;White, M. Christina;Gormisky, Paul E. US9925528, 2018, B2 Location in patent:Page/Page column 36-37; 47-48
1073-13-8
157 suppliers
$8.00/1g

4255-62-3
199 suppliers
$10.00/1g

590-66-9
21 suppliers
$76.00/1g

2979-19-3
227 suppliers
$6.00/1g

1193-47-1
93 suppliers
$87.00/250mg

4255-62-3
199 suppliers
$10.00/1g
