
4,4-DIPHENYLCYCLOHEXANOL synthesis
- Product Name:4,4-DIPHENYLCYCLOHEXANOL
- CAS Number:42420-85-9
- Molecular formula:C18H20O
- Molecular Weight:252.35
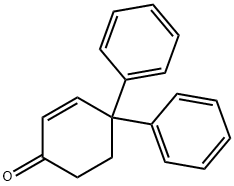
4528-64-7
41 suppliers
$108.00/1g

42420-85-9
21 suppliers
$130.00/1g
Yield:42420-85-9 96%
Reaction Conditions:
Stage #1: 4,4-diphenyl-2-cyclohexen-1-onewith palladium 10% on activated carbon;hydrogen in tetrahydrofuran; under 760.051 Torr; for 20 h;Sealed tube;
Stage #2: with sodium tetrahydroborate;sodium hydroxide in tetrahydrofuran;water at 0 - 20; for 2 h;
Steps:
1.3 1.3 Synthesis of 4,4-diphenylcyclohexanol
1.3 Synthesis of 4,4-diphenylcyclohexanol. To a solution of 4,4-diphenyl-2- cyclohexen-l-one (3.40 g, 13.7 mmol) in THF (30 mL) was added 10% palladium on carbon (140 mg). The reaction vessel was flushed with hydrogen gas, and the system was sealed under a hydrogen atmosphere (1 atm) and stirred vigorously for 20 h. The catalyst was removed by filtration through Celite, washing with THF (15 mL), and the filtrate was chilled to 0 °C in an ice bath. Sodium borohydride (0.26 g, 6.85 mmol) was dissolved in 0.1 M aqueous NaOH (7 mL) in a separate flask and chilled to 0 °C, then added dropwise to the organic solution. The system was warmed to room temperature and stirred for 2 h. The reaction was then chilled to 0 °C, quenched with 2 M aqueous HC1 (12 mL) and diluted with cold H20 (100 mL). The system was stirred at 0 °C for 30 min, over which time a white precipitate formed. The precipitate was isolated by filtration, washed with cold H20 and dried under reduced pressure to yield the product (3.32 g, 13.2 mmol, 96%> over two steps) as a white solid. Rf 0.26 (4: 1 hexanes/EtOAc). Spectral data matched those reported previously (Amedio Jr. et al. , 1998, Synth. Comm. 28, 3895).
References:
WO2015/142701,2015,A1 Location in patent:Page/Page column 30
947-91-1
117 suppliers
$24.73/1gm:

42420-85-9
21 suppliers
$130.00/1g

4528-68-1
17 suppliers
$498.47/5MG

42420-85-9
21 suppliers
$130.00/1g