
4-[(4-FLUOROBENZYL)OXY]PIPERIDINE synthesis
- Product Name:4-[(4-FLUOROBENZYL)OXY]PIPERIDINE
- CAS Number:81151-35-1
- Molecular formula:C12H16FNO
- Molecular Weight:209.26

1121587-29-8
2 suppliers
inquiry
![4-[(4-FLUOROBENZYL)OXY]PIPERIDINE](/StructureFile/ChemBookStructure21/GIF/CB8799418.gif)
81151-35-1
8 suppliers
inquiry
Yield:81151-35-1 0.5 g
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 1 h;
Steps:
247.B
B. To a solution of tert-butyl 4-((4-fluorobenzyl)oxy)piperidine-1-carboxylate (800 mg, 2.59 mmol) in THF (2 mL) was added con hydrogen chloride (2 mL). The reaction was stirred at ambient temperature for 1 h. Saturated aqueous NaHCO3 (30 mL) and DCM (200 mL) were added to the reaction vessel and the resulting biphasic mixture was transferred to a separatory funnel. The layers were separated and the organic phase was washed with saturated aqueous NaCl (2 x 20 mL). The combined organics were dried over anhydrous Na2SO4, filtered and concentrated in vacuo to provide 4-((4-fluorobenzyl)oxy)piperidine (0.5 g, 2.39 mmol) as a green oil.
References:
WO2018/26371,2018,A1 Location in patent:Paragraph 0311

109384-19-2
469 suppliers
$5.00/1g
![4-[(4-FLUOROBENZYL)OXY]PIPERIDINE](/StructureFile/ChemBookStructure21/GIF/CB8799418.gif)
81151-35-1
8 suppliers
inquiry
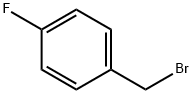
459-46-1
327 suppliers
$10.00/1g
![4-[(4-FLUOROBENZYL)OXY]PIPERIDINE](/StructureFile/ChemBookStructure21/GIF/CB8799418.gif)
81151-35-1
8 suppliers
inquiry