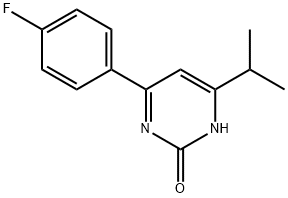
4-(4-Fluorophenyl)-6-isopropylpyrimidin-2-ol synthesis
- Product Name:4-(4-Fluorophenyl)-6-isopropylpyrimidin-2-ol
- CAS Number:894787-95-2
- Molecular formula:C13H13FN2O
- Molecular Weight:232.25
Yield:894787-95-2 64%
Reaction Conditions:
with hydrogenchloride in ethanol;isopropyl alcohol; for 40 h;Product distribution / selectivity;Heating / reflux;
Steps:
A 5M to 6M solution of hydrogen chloride in isopropanol (38 mL, 194 mmoles) was added to a stirred mixture of urea (7.78 g, 129.6 mmoles) and 1-(4-fluorophenyl)-4-methylpentane-1,3-dione (8.43 g, 32.4 mmoles) in ethanol (49 mL). The reaction mixture was refluxed for 40 hours and then cooled to -6° C. The resultant precipitate was collected by filtration and washed with diethyl ether (20 mL). The solid was added to water (60 mL) and saturated aqueous sodium bicarbonate solution (10 mL). Further solid sodium bicarbonate (16.4 g, 195 mmoles) was added portionwise. The mixture was diluted with acetone (40 mL) and ethyl acetate (80 mL). The organic phase was separated and aqueous phase was extracted 2:1 ethyl acetate/acetone (3×120 mL). The organic phases were combined, washed with brine (30 mL), dried with anhydrous magnesium sulfate and concentrated in vacuo to yield 4.8 g of 4-(4-fluorophenyl)-6-isopropylpyrimidin-2-ol (64% yield); 1H NMR (400 MHz) (CDCl3) δ TMS: 1.41 (6H, d, J=6.90 Hz), 3.08 (1H, m), 6.69 (1H, s), 7.17 (2H, dd, J=8.60 Hz, J=8.60 Hz), 8.14 (2H, dd, J=6.65 Hz, J=6.65 Hz), 13.57 (1H, br. s). Mp: 215-217° C. HRMS calculated for C13H13N2OF. 232.1012, found 232.0963; used in subsequent reaction without further purification.
References:
US2008/207903,2008,A1 Location in patent:Page/Page column 17

563-80-4
294 suppliers
$18.00/25mL
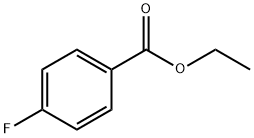
451-46-7
285 suppliers
$5.00/5g
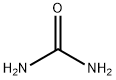
57-13-6
701 suppliers
$5.00/5g

894787-95-2
5 suppliers
inquiry
