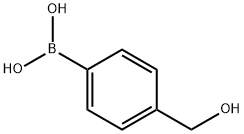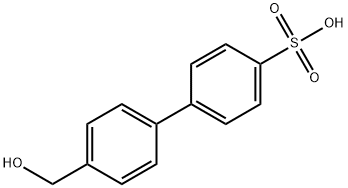
4-(4-Hydroxymethylphenyl)phenylsulfonic acid synthesis
- Product Name:4-(4-Hydroxymethylphenyl)phenylsulfonic acid
- CAS Number:74758-55-7
- Molecular formula:C13H12O4S
- Molecular Weight:264.3
Yield:74758-55-7 19%Spectr.
Reaction Conditions:
with C31H27N5O4PdS2;potassium carbonate in water at 100; for 12 h;Catalytic behavior;
Steps:
2.4. Suzuki-Miyaura coupling experiment
General procedure: In a typical catalytic reaction, (4-bromophenyl)methanol (0.19 g,1.0 mmol), benzeneboronic acid (0.15 g, 1.2 mmol), K3CO3 (0.14 g,1.0 mmol) and preformed complex (0.2 mol % relative to (4-bromophenyl)methanol) were added together in a 10mL roundbottomflask equipped with magnetic stirrer bar. In the case of insitu, 0.2 mol % Pd(AcO)2 and 0.4 mol % ligand were used relative to(4-bromophenyl)methanol. Water (4 mL) was added and themixture refluxed on an oil bath set at 100 C for the desired reactiontime. An aliquot of the reaction mixture was taken into a 10 mLconical flask which was dried under vacuum to exclude the solvents.The residue was subjected to 1H NMR measurement usingdeuterated DMSO. The reactivity of the catalyst was evaluated bycomparison of the NMR signals for the methylene (eCH2e) protonsof (4-bromophenyl)methanol at dz4.4 ppm with that of the correspondingbiphenyl product at dz4.6 ppm. The yields wereestimated by determining the integral of the product peak as apercentage of the sum of all observed methylene signals [57].
References:
Oloyede, Hammed Olawale;Orighomisan Woods, Joseph Anthony;G?rls, Helmar;Plass, Winfried;Eseola, Abiodun Omokehinde [Journal of Molecular Structure,2020,vol. 1199,art. no. 127030]