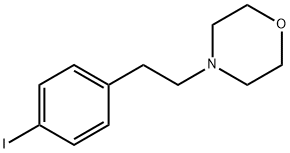
4-(4-iodophenethyl)morpholine synthesis
- Product Name:4-(4-iodophenethyl)morpholine
- CAS Number:100839-46-1
- Molecular formula:C12H16INO
- Molecular Weight:317.17
![Morpholine, 4-[(4-iodophenyl)acetyl]-](/CAS/20210111/GIF/364793-89-5.gif)
364793-89-5
4 suppliers
inquiry

100839-46-1
5 suppliers
inquiry
Yield:100839-46-1 54.3%
Reaction Conditions:
Stage #1: 2-(4-iodophenyl)-1-morpholin-4-ylethanonewith sodium tetrahydroborate;boron trifluoride diethyl etherate in tetrahydrofuran at 0 - 20;
Stage #2: with water in tetrahydrofuran at 0 - 20; for 16 h;
Stage #3: with water at 0;
Steps:
4- [2-(4-Iodo-phenyl)-ethyl] -morpholine
4- [2-(4-Iodo-phenyl)-ethyl] -morpholine: BF3.Et20 (6.43 g, 45.3 mmol) was added to a solution of 2-(4-iodo-phenyl)-l-morpholin-4-yl- ethanone (3.0 g, 9.06 mmol) in THF (75 mL) at 0 °C, stirred for 30 min, then NaBFL, (1.72 g, 45.3 mmol) was added at 0 °C. The reaction mixture was slowly warmed to RT. After 16 h the reaction mixture was cooled to 0 °C, quenched with ice-cold water, extracted with EtOAc (2 x 100 mL). The organic layer was washed with brine solution (50 mL), dried over anhydrous Na2S04 and solvent was evaporated under reduced pressure. The crude compound was purified by column chromatography using 100-200 mesh silica gel, eluted with 20-30% EtOAc in pet- ether to afford 1.56 g (Yield: 54.3%) of the title compound as a yellow solid. 1H NMR (DMSO-dg, 400 MHz, TMS) δ: 7.64-7.62 (2H, d), 7.07-7.05 (2H, d), 4.11-4.07 (2H, q), 3.58 (4H, br, s), 2.67 (2H, br, s), 2.46 (4H, br, s).
References:
WO2014/106612,2014,A1 Location in patent:Page/Page column 31-32
1798-06-7
174 suppliers
$6.00/1g

100839-46-1
5 suppliers
inquiry