
4-(4-ISOPROPYLPIPERAZIN-1-YL)BENZALDEHYDE synthesis
- Product Name:4-(4-ISOPROPYLPIPERAZIN-1-YL)BENZALDEHYDE
- CAS Number:197638-78-1
- Molecular formula:C14H20N2O
- Molecular Weight:232.32
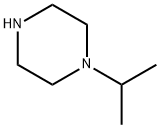
4318-42-7
235 suppliers
$6.00/250mg

459-57-4
577 suppliers
$6.00/25g

197638-78-1
9 suppliers
$303.00/100mg
Yield:197638-78-1 84%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide;Reflux;
Steps:
3.1.1. Synthesis of 4-Substituted Benzaldehydes 1a-1d
General procedure: A solution of 4-fluorobenzaldehyde (0.017 mol, 1.82 mL) and an appropriate secondary aminein DMF (10 mL) was refluxed (0.017 mol) in the presence of potassium carbonate (K2CO3, 0.017 mol,2.35 g). After completion of reaction, the mixture was cooled with ice-water (10 mL) and extractedwith ethyl acetate (3 20 mL). The ethyl acetate phases were combined, and the remaining productwas collected after evaporation of the solvent. The following compounds were prepared in this way:4-(4-methylpiperazin-1-yl)benzaldehyde (1a), CAS No:27913-99-1, Yield 82%, m.p. = 55-57 °C (measured),m.p. = 58-60 °C (reported) [60]; 4-(4-ethylpiperazin-1-yl)benzaldehyde (1b), CAS No:197638-76-9,Yield 79%, obtained as an oil; 4-(4-isopropylpiperazin-1-yl)benzaldehyde (1c), CAS No:197638-78-1,Yield 84%, obtained as an oil and 4-(4-cylopropylpiperazin-1-yl)benzaldehyde (1d), CAS No:1292778-23-4,Yield 78%, obtained as an oil.
References:
Tokg?z, Gamze;?zkay, ümide Demir;Osmaniye, Derya;Yücel, Nazl? Turan;Can, ?zgür Devrim;Kaplanc?kl?, Zafer As?m [Molecules,2018,vol. 23,# 11,art. no. 2881]
![Benzonitrile, 4-[4-(1-methylethyl)-1-piperazinyl]-](/CAS/20210305/GIF/186650-79-3.gif)
186650-79-3
0 suppliers
inquiry

197638-78-1
9 suppliers
$303.00/100mg
68104-63-2
128 suppliers
$10.00/250mg

197638-78-1
9 suppliers
$303.00/100mg