
4-(4-METHYLPHENOXY)PHENOL synthesis
- Product Name:4-(4-METHYLPHENOXY)PHENOL
- CAS Number:35094-91-8
- Molecular formula:C13H12O2
- Molecular Weight:200.23
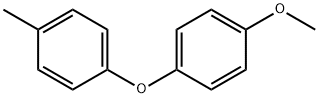
3402-85-5
7 suppliers
inquiry

35094-91-8
15 suppliers
$90.00/100mg
Yield:35094-91-8 95%
Reaction Conditions:
Stage #1: 1-methoxy-4-(p-tolyloxy)benzenewith boron tribromide in dichloromethane at 0 - 20;Inert atmosphere;
Stage #2: with water in dichloromethane;
Steps:
9.1.2
DemethylationUnder inert atmosphere, the methoxyether derivative previously prepared was dissolved in dichloromethane. The reaction mixture was cooled to 0° C. before adding a molar solution of boron tribromide in dichloromethane (2eq) drop by drop. The reaction mixture was stirred at 0° C. for 30 minutes, then at room temperature for 3 hours. The mixture was then partitioned between water and dichloromethane. The organic layer was washed with a sodium hydroxide 2N solution. The aqueous layer was acidified to pH 1 and extracted with dichloromethane. The organic layers were combined, dried over sodium sulfate, and evaporated under reduced pressure.9.1.2 4-(p-tolyloxy)phenol Prepared following the demethylation method previously described (Method 9A) using 1-methoxy-4-(p-tolyloxy)benzene (example 9.1.1). The product was obtained as a beige solid. Yield: 95% Rf (cyclohexane/ethyl acetate 8/2): 0.32 NMR 1H (CDCl3): 2.30 (s, 3H); 4.86 (s, 1H); 6.72-6.95 (m, 6H); 7.10 (d, 2H, J=8.2 Hz).
References:
US2010/4159,2010,A1 Location in patent:Page/Page column 55-56
5720-05-8
426 suppliers
$5.00/1g
106-51-4
478 suppliers
$10.00/5g

35094-91-8
15 suppliers
$90.00/100mg

61343-83-7
42 suppliers
$57.00/1g

35094-91-8
15 suppliers
$90.00/100mg

459-57-4
580 suppliers
$6.00/25g

35094-91-8
15 suppliers
$90.00/100mg
95872-23-4
0 suppliers
inquiry

35094-91-8
15 suppliers
$90.00/100mg