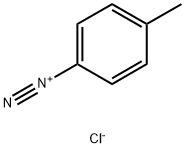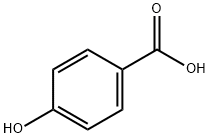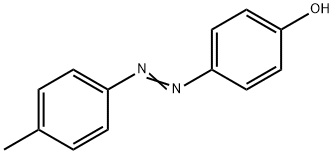
4-(4-Methylphenylazo)phenol synthesis
- Product Name:4-(4-Methylphenylazo)phenol
- CAS Number:2497-33-8
- Molecular formula:C13H12N2O
- Molecular Weight:212.25
Yield:2497-33-8 96%
Reaction Conditions:
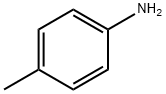
Stage #1: p-toluidinewith hydrogenchloride in lithium hydroxide monohydrate at 0;
Stage #2: with NaNO2 in lithium hydroxide monohydrate at 0 - 5; for 0.25 h;
Stage #3: phenolwith anhydrous sodium carbonate;sodium hydroxide in lithium hydroxide monohydrate at 0 - 5;
Steps:
Synthesis of p-hydroxy-p’-methyl-azobenzene
35 mL of concentrated hydrochloric acid and 35 mL water were added to 21.4 g(0.2 mol) of p-toluidine (p-methylaniline) and the resulting solution was cooled down to 0°C. Then a solution of 15 g (0.2 mol) of sodium nitrite in30 mL water was added drop-wise and the mixture was stirred at a temperature between 0 and 5°C for 15 min. Second step, the resulting clear solution of diazonium salt was slowly added to a solution of28.2 g (0.3 mol) phenol, 12 g (0.3 mol) NaOH,32 g (0.3 mol) Na2CO3 in 150 ml water at 0-5°C.Sometimes it was necessary to add extra NaOH to avoid formation of foam. After complete addition,the mixture was stirred for 1-2 h at 0-5°C. The yellow-orange colored precipitate was filtered off,washed with water and then dried in a vacuum oven at 100°C. Melting point: 149-152°C. Yield:96%.
References:
Sava, Ion;K?pnick, Thomas [Revue Roumaine de Chimie,2014,vol. 59,# 6-7,p. 585 - 592]
106-51-4
478 suppliers
$10.00/5g

2497-33-8
14 suppliers
$77.00/1G
106-49-0
391 suppliers
$5.00/5G

2497-33-8
14 suppliers
$77.00/1G