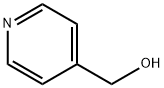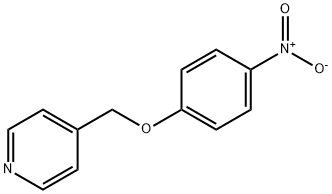
4-[(4-nitrophenoxy)methyl]pyridine synthesis
- Product Name:4-[(4-nitrophenoxy)methyl]pyridine
- CAS Number:252918-98-2
- Molecular formula:C12H10N2O3
- Molecular Weight:230.22
Yield:-
Reaction Conditions:
Stage #1: pyridine-4-methanolwith potassium hydroxide;Aliquat 336 at 20; for 0.0833333 h;
Stage #2: 4-chlorobenzonitrile at 20 - 80; for 2.08333 h;
Steps:
4- (4-Nitro-phenoxymethyl)-pyridine
4-hydroxymethyl-pyridine (2.23 g, 20 [MMOL)] is added to a suspension of KOH (1.32 g, 20 [MMOL)] and Aliquat 336 (0.937 mL). After stirring for 5 min at rt, 1-chloro-4-nitro-benzene (2.68 g, 16.7 [MMOL)] is added. The resulting reaction mixture is further stirred at rt for 5 min and then at 80 [C] for 2 h. The reaction mixture is filtered over silica gel (15 [G),] concentrated under reduced pressure, and flash chronatographed (silica gel, 3 x 50 cm, [ACETONE/CH2CI2] = 5: [95-] 15: 85) to give the title compound as a yellow solid : M+H = 231.0 [;'H-NMR] (400 MHz, DMSO-d6) : 8.58 (d, 6.5 Hz, 2H, pyrimidinyl), 8.22 (d, 8.5 Hz, 2H, phenyl-NO2), 7.44 (d, 6.5 Hz, pyrimidinyl), 7.21 (d, 8.5 Hz, 2H, phenyl-NO2), 5.34 (s, 2H, CH2); Rf(acetone/CH2Cl2 = 15: 85): 0.32 ; HPLC (System 1): 3.40 min.
References:
WO2003/99771,2003,A2 Location in patent:Page 84