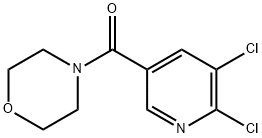
4-[(5,6-dichloropyridin-3-yl)carbonyl]morpholine synthesis
- Product Name:4-[(5,6-dichloropyridin-3-yl)carbonyl]morpholine
- CAS Number:919784-85-3
- Molecular formula:C10H10Cl2N2O2
- Molecular Weight:261.1

110-91-8
677 suppliers
$9.00/1g
54127-29-6
35 suppliers
$29.00/100mg
![4-[(5,6-dichloropyridin-3-yl)carbonyl]morpholine](/CAS/20200401/GIF/919784-85-3.gif)
919784-85-3
9 suppliers
inquiry
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 18 h;
Steps:
Oxalyl chloride (3.2 mL; 36.0 mmol) was added to a solution of 5,6 dichloronicotinic acid (5.76 g; 30.0 mmol) and 4M hydrogen chloride in dioxane (7.5 mL; 30 mmol) in DCM (50 mL). The mixture was stirred at RT for 18 hours, the DCM evaporated in vacuo, the residue azeotroped with toluene (2 x 15 mL) and added to a solution of morpholine (3.1 mL; 36.0 mmol) and triethylamine (10.0 mL; 72 mmol) in DCM (150 mL). The mixture was stirred at RT for 18 hours, the DCM evaporated in vacuo, and the residue partitioned between water (75 mL) and ethyl acetate (100 mL). The organic layer was washed with brine, dried (MgSO4) and evaporated to a residue which was chromatographed on silica, eluting with 50% ethyl acetate in isohexane, to give the desired compound (7.1 g). 1H NMR δ (CDCl3): 3.3 - 3.8 (br, 8H), 7.8 (s, IH), 8.25 (s, IH); m/z 261 (M+H)+.
References:
WO2007/7041,2007,A1 Location in patent:Page/Page column 208