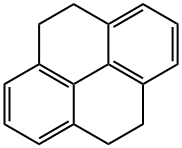
4,5,9,10-TETRAHYDROPYRENE synthesis
- Product Name:4,5,9,10-TETRAHYDROPYRENE
- CAS Number:781-17-9
- Molecular formula:C16H14
- Molecular Weight:206.28

129-00-0
325 suppliers
$9.00/1g

781-17-9
61 suppliers
$50.00/100mg
Yield:781-17-9 90%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in tetrahydrofuran;methanol at 90; under 7500.75 Torr; for 168 h;Autoclave;
Steps:
4,5,9,10-Tetrahydropyrene 2
A solution of pyrene 1 (2.5 g, 12.2 mmol) in a THF:MeOH mixture (1:5) (60 mL) was hydrogenated over 10 % Pd/C (850 mg) in an autoclave at 90°C and with H2, at 10 Bar, for 7 days. The reaction mixture was filtered through Celite and washed several times with THF. The filtrate was concentrated, then diluted with dichloromethane (2 x 25 mL) and washed with water (2 x 50 mL) and brine. The organic extract was dried over anhydrous MgSO4, filtered, and evaporated. The crude residue was crystallized from DCM:EtOH (1:9) to give the product as a shiny white crystalline solid (2.26 g, 10.9 mmol, 90 %); mp. 129-131°C (lit,14 mp 133°C); 1H NMR (400 MHz, CDCl3): δ 7.11 (m, 6H), 2.91 (s, 8H); EIMS calculated for [C16H14]: 206.11; Found: 206.10; 1,2,3,6,7,8-Hexahydropyrene 3 (present in the mixture): 1H NMR (400 MHz, CDCl3): δ 7.14 (s, 4H), 3.09 (d, 8H, J=5.3Hz), 2.07 (d, 4H, J=5.0Hz).
References:
Cabral, Lília I.L.;Henriques, Marta S.C.;Paixão, José A.;Cristiano, Maria L.S. [Tetrahedron Letters,2017,vol. 58,# 48,p. 4547 - 4550] Location in patent:supporting information
6628-98-4
2 suppliers
inquiry

781-17-9
61 suppliers
$50.00/100mg

129-00-0
325 suppliers
$9.00/1g

6628-98-4
2 suppliers
inquiry

781-17-9
61 suppliers
$50.00/100mg
1732-13-4
69 suppliers
$89.00/1g
5385-37-5
7 suppliers
$502.43/5MG