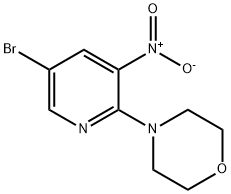
4-(5-BROMO-3-NITRO-PYRIDIN-2-YL)-MORPHOLINE synthesis
- Product Name:4-(5-BROMO-3-NITRO-PYRIDIN-2-YL)-MORPHOLINE
- CAS Number:505052-64-2
- Molecular formula:C9H10BrN3O3
- Molecular Weight:288.1

110-91-8
677 suppliers
$9.00/1g
67443-38-3
362 suppliers
$5.00/1g

505052-64-2
23 suppliers
$424.00/10g
Yield:-
Reaction Conditions:
in dimethyl sulfoxide at 23; for 20 h;
Steps:
63
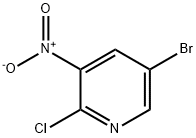
To a vial containing 5-bromo-2-chloro-3-nitropyridine (0.99 g, 4.16 mmol) in DMSO (4.0 mL) was added morpholine (0.8 mL, 9.19 mmol) dropwise. The reaction was stirred at 23° C. and monitored with TLC and LC-MS. After 20 h, LC-MS showed that the reaction was complete, then the mixture was diluted with water. After extracting three times with EtOAc, the organic layers were combined then washed with brine and dried over anhydrous magnesium sulfate. After filtration, the mixture was concentrated in vacuo to afford a yellow-orange solid as 4-(5-bromo-3-nitropyridin-2-yl)morpholine. 1H NMR (400 MHz, DMSO-d6) δ ppm 8.53 (1H, d, J=2.3 Hz), 8.47 (1H, d, J=2.3 Hz), 3.70 (4H, m), 3.41 (4H, m).
References:
US2010/331293,2010,A1 Location in patent:Page/Page column 68