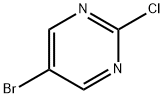
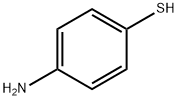
4-[(5-BROMOPYRIMIDIN-2-YL)THIO]ANILINE synthesis
- Product Name:4-[(5-BROMOPYRIMIDIN-2-YL)THIO]ANILINE
- CAS Number:849035-61-6
- Molecular formula:C10H8BrN3S
- Molecular Weight:282.16
Yield:849035-61-6 74%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 120; for 2.5 h;
Steps:
A solution of 5-bromo-2-chloropyrimidine (5 g, 0.026 mol), 4-aminothiophenol (3.24 g, 0.026 mol), and K2CO3 (7.14 g, 0.052 mol) in dry DMSO (50 mL) was stirred at 120° C. for 2.5 hours under N2. After cooling, the reaction mixture was poured into water and extracted with ethyl acetate. The organic layer was washed with water and saturated brine and then dried. The solvent was evaporated to give a residue that was purified by silica gel flash column chromatography (ethyl acetate-hexane 1:3) to give 4-(5-bromopyrimidin-2-ylsulfanyl)phenylamine (4n), yield 74%. 1H NMR (400 MHz, CDCl3): δ 8.51 (s, 2H), 7.37 (d, J) 8.0 Hz, 2H), 6.72 (d, J) 8.0 Hz, 2H), 2.63 (s, 2H); EI-MS m/z 281 [M]+, 283 [M+2]+.A solution of 2-nitrobenzoyl isocyanate (3 g, 0.016 mol) in dry 1,4-dioxane (15 mL) was added dropwise to a solution of 4n (2.93 g, 0.01 mol) in dry 1,4-dioxane (15 mL) with stirring at room temperature. The reaction mixture was stirred for 18 hours and then diluted with water. The precipitated solid was collected by filtration and washed with water. The solid was dissolved in ethyl acetate, and the organic layer was washed with water 2-3 times, dried, and concentrated to give 1-[4-(5-bromopyrimidin-2-ylsulfanyl)-phenyl]-3-(2-nitrobenzoyl)urea (6n), yield 92%. 1H NMR (400 MHz, DMSO-d6): δ 11.32 (br, s, 1H), 10.35 (br, s, 1H), 8.78 (s, 2H), 8.22 (m, 1H), 7.91 (m, 1H), 7.77 (m, 2H), 7.67 (m, 2H), 7.57 (m 2H); EI-MS m/z 473 [M]+, 475 [M+2]+; HRMS calculated for C18H12BrN5O4SNa [M+Na]+: 495.9685, found: 495.9701. Anal. (C18H12BrN5O4S) C, H, N.
References:
US2009/99218,2009,A1 Location in patent:Page/Page column 20