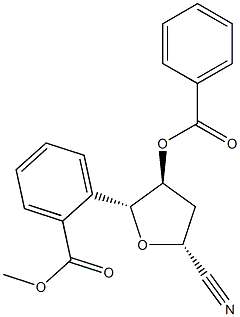
β-4,5-dibenzoyl-2-cyano-D-deoxyribrate synthesis
- Product Name:β-4,5-dibenzoyl-2-cyano-D-deoxyribrate
- CAS Number:170983-98-9
- Molecular formula:C20H21NO3
- Molecular Weight:323.39
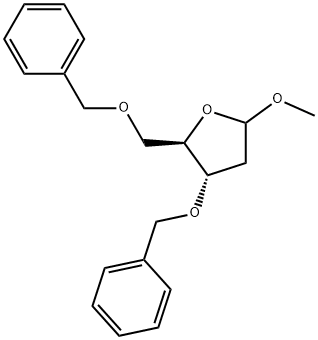
132487-16-2
18 suppliers
$350.00/1 g
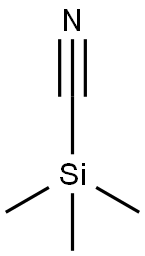
7677-24-9
380 suppliers
$19.00/5g

170983-98-9
2 suppliers
inquiry
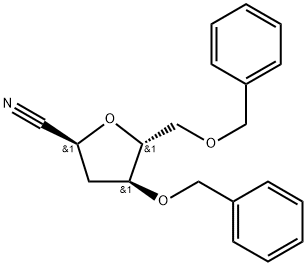
226232-74-2
3 suppliers
inquiry
Yield: 67% , 25%
Reaction Conditions:
with trimethylsilyl trifluoromethanesulfonate in dichloromethane at 20; for 2 h;Inert atmosphere;
Steps:
1β-Cyano-3,5-di-O-benzyl-1,2-dideoxy-D-ribofuranose (4) and 1α-cyano-3,5-di-O-benzyl-1,2-dideoxy-D-ribofuranose (5)
Under a nitrogen atmosphere, TMSOTf (8.10 mL, 29.0 mmol) in anhydrous CH2Cl2 (200 mL) was added toa solution of 2-deoxy-3,5-di-O-benzyl-D-ribofuranose 37 (6.34 g, 19.3 mmol) and TMSCN (4.70 mL, 58.0mmol) in anhydrous CH2Cl2 (200 mL), and the mixture was stirred at room temperature for 2 h. Afteraddition of sat. NaHCO3 aq., the mixture was extracted with CH2Cl2. The extracts were washed with waterand brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flashsilica gel column chromatography (n-hexane/AcOEt = 5:1) to give compounds 4 (1.57 g, 25%) and 5 (4.17g, 67%), respectively. Compound 4: A colorless oil. []D 30.1 (c 0.65, CHCl3, 29 C). IR max (KBr) 3031,2925, 2856, 2245, 1455, 1098 cm-1. 1H NMR (400 MHz, CDCl3) 2.40-2.43 (2H, m), 3.50 (1H, dd, J = 5.0,10.5 Hz), 3.57 (1H, dd, J = 4.0, 10.5 Hz), 4.18-4.22 (1H, m), 4.23-4.26 (1H, m), 4.48 (1H, d, J = 12.0 Hz),4.49 (1H, d, J = 12.0 Hz), 4.53 (1H, d, J = 12.0 Hz), 4.58 (1H, d, J = 12.0 Hz), 4.78 (1H, dd, J = 7.5, 8.0 Hz),7.25-7.37 (10H, m). 13C NMR (101 MHz, CDCl3) 37.6, 65.7, 70.1, 71.4, 73.6, 80.0, 84.7, 118.8, 127.7,127.7, 127.8, 128.0, 128.4, 128.5, 137.3, 137.7. HRMS (MALDI) m/z calcd for C20H21NNaO3 [M+Na]+:346.1414; found, 346.1414. Compound 5: A colorless oil. []D 64.7 (c 1.13, CHCl3, 29 C). IR max (KBr)3031, 2864, 2239, 1454, 1092 cm-1. 1H NMR (400 MHz, CDCl3) 2.38-2.41 (2H, m), 3.51 (2H, d, J = 4.0Hz), 4.15-4.18 (1H, m), 4.36-4.39 (1H, m), 4.49 (1H, d, J = 12.0 Hz), 4.50 (1H, d, J = 12.0 Hz), 4.51 (1H,d, J = 12.0 Hz), 4.58 (1H, d, J = 12.0 Hz), 4.87 (1H, dd, J = 4.0, 4.0 Hz), 7.25-7.38 (10H, m). 13C NMR (101MHz, CDCl3) 37.2, 66.1, 69.8, 71.3, 73.5, 79.3, 84.8, 119.0, 127.6, 127.6, 127.8, 128.4, 128.5, 137.4,137.6. HRMS (MALDI) m/z calcd for C20H21NNaO3 [M+Na]+: 346.1414; found, 346.1411.
References:
Hari, Yoshiyuki;Kashima, Satoshi;Matsuda, Yuya;Sakata, Akihiro;Takamine, Ryutaro;Ijitsu, Shin;Obika, Satoshi [Heterocycles,2015,vol. 90,# 1,p. 432 - 441]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

170983-98-9
2 suppliers
inquiry

132487-16-2
18 suppliers
$350.00/1 g

7677-24-9
380 suppliers
$19.00/5g

170983-98-9
2 suppliers
inquiry

6160-49-2
0 suppliers
inquiry

7677-24-9
380 suppliers
$19.00/5g

170983-98-9
2 suppliers
inquiry

226232-74-2
3 suppliers
inquiry

132487-16-2
18 suppliers
$350.00/1 g

170983-98-9
2 suppliers
inquiry