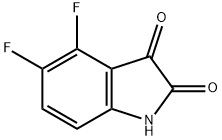
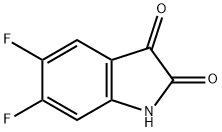
4,5-Difluoroindoline-2,3-dione synthesis
- Product Name:4,5-Difluoroindoline-2,3-dione
- CAS Number:169037-22-3
- Molecular formula:C8H3F2NO2
- Molecular Weight:183.11
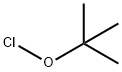
507-40-4
129 suppliers
$144.00/25g
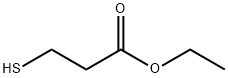
5466-06-8
165 suppliers
$66.00/25g
3863-11-4
327 suppliers
$10.00/25g

169037-22-3
4 suppliers
inquiry
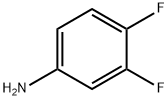
774-47-0
74 suppliers
$8.00/250mg
Yield:774-47-0 31% and 4%
Reaction Conditions:
with hydrogenchloride;N-chloro-succinimide;trifluoroborane diethyl ether;triethylamine in tetrahydrofuran;diethyl ether;dichloromethane;chloroform;water;
Steps:
1 Preparation of 5,6-difluoroisatin and 4,5-difluoroisatin STR9
EXAMPLE 1 Preparation of 5,6-difluoroisatin and 4,5-difluoroisatin STR9 To a solution of 3,4-difluoroaniline (12.98 g, 0.100 mol) in 325 mL of methylene chloride at -65° C. was added a solution of t-butylhypochlorite (10.86 g, 0.100 mol) in 52 mL of methylene chloride. The mixture was stirred for 10 min. A solution of ethyl thiomethylacetate (13.49 g, 0.100 mol) in 65 mL of methylene chloride was added dropwise to the mixture and stirred at -65° C. for 1 h. Triethylamine (10.17 g, 0.100 mol) in 65 mL of methylene chloride was added and the reaction mixture was warmed to room temperature and stirred for 3 h. Water was added and the methylene chloride layer was separated and concentrated under reduced pressure to yield an oil. The resulting oil was diluted with 300 mL of diethyl ether and 80 mL of 2N HCl, and stirred for 24 h. A precipitate was formed, filtered and washed with 50 mL of diethyl ether to give a mixture of 5,6- and 4,5-difluoro-3-thiomethyloxindoles in 70% yield. The crude oxindoles (11.64 g, 0.054 mol) were reacted with N-chlorosuccinimide (7.26 g, 0.05 mol) in 500 mL of chloroform at room temperature for 1 h. The reaction mixture was concentrated and the resulting residue was dissolved in 70 mL of THF. To this solution was added red mercury (II) oxide (11.78 g, 0.054 mol), boron trifluoride etherate (7.72 g, 0.05 mol), and 400 mL of aqueous 20% THF. The slurry was stirred for 3 h, diluted with 1000 mL of chloroform and filtered through celite. The resulting solids were washed with chloroform and the chloroform layer was separated and concentrated. Chromatography on silica gel eluding with 1% isopropyl alcohol:chloroform gave 5,6-difluoroisatin (Saul Kadin, U.S. Pat. No. 4,721,712) and 4,5-difluoroisatin in 31% and 4% yield, respectively. 4,5-Difluoroisatin: mp 140° C. (dec); 1 H NMR (300 MHz, DMSO-d6) δ 11.25 (s, 1H), 7.7 (dd, 1H), 6.7 (dd, 1H).
References:
US5441955,1995,A