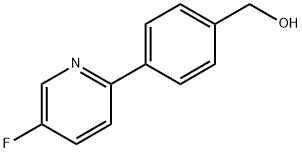
4-(5-Fluoro-2-pyridinyl)benzeneMethanol synthesis
- Product Name:4-(5-Fluoro-2-pyridinyl)benzeneMethanol
- CAS Number:1257426-54-2
- Molecular formula:C12H10FNO
- Molecular Weight:203.21
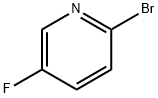
41404-58-4
399 suppliers
$5.00/1g
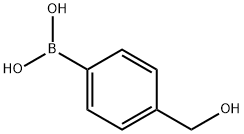
59016-93-2
312 suppliers
$5.00/1g

1257426-54-2
18 suppliers
$400.39/250mg
Yield:1257426-54-2 46%
Reaction Conditions:
with sodium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in ethanol;water;toluene at 89; for 3.33333 h;Inert atmosphere;
Steps:
2.AA
A stirred mixture of 4-(hydroxymethyl)phenylboronic acid (34) (501 mg, 3.30 mmol) and Pd(dppf)Cl2 (338 mg, 0.462 mmol) in toluene (36 mL) and EtOH (18 mL) was degassed for 15 min (vacuum pump) and then N2 was added. An aqueous solution of 2M Na2CO3 (9 mL, 18 mmol) was added by syringe and the stirred mixture was again degassed for 15 min, and then N2 was added. 2-Bromo-5-fluoropyridine (110) (1.44 g, 8.18 mmol) was added by syringe and the resulting mixture was stirred at 89 0C for 200 min. The cooled mixture was then diluted with aqueous NaHCO3 (100 mL) and extracted with CH2Cl2 (5x 100 mL). The extracts were evaporated to dryness and the residue was chromatographed on silica gel. Elution with CH2Cl2 and 0-25% Et2O/petroleum ether firstly gave foreruns, and then further elution with 33-50% Et2O/petroleum ether gave [4-(5-fluoro-2-pyridinyl)phenyl]methanol (111) (307 mg, 46%) as a cream solid (following pentane trituration): mp 100-101 0C; 1H NMR (CDCl3) δ 8.54 (d, J = 2.9 Hz, 1 H), 7.94 (dt, J = 8.4, 1.9 Hz, 2 H), 7.72 (ddd, J = 8.8, 4.2, 0.5 Hz, 1 H), 7.50- 7.43 (m, 3 H), 4.76 (d, J = 6.0 Hz, 2 H), 1.69 (t, J = 6.0 Hz, 1 H); HRESIMS calcd for C12HnFNO mlz [M + H]+ 204.0819, found 204.0824.
References:
WO2011/14774,2011,A1 Location in patent:Page/Page column 52