
4-(5-methyl-1,3,4-oxadiazol-2-yl)piperidine synthesis
- Product Name:4-(5-methyl-1,3,4-oxadiazol-2-yl)piperidine
- CAS Number:161609-79-6
- Molecular formula:C8H13N3O
- Molecular Weight:167.21
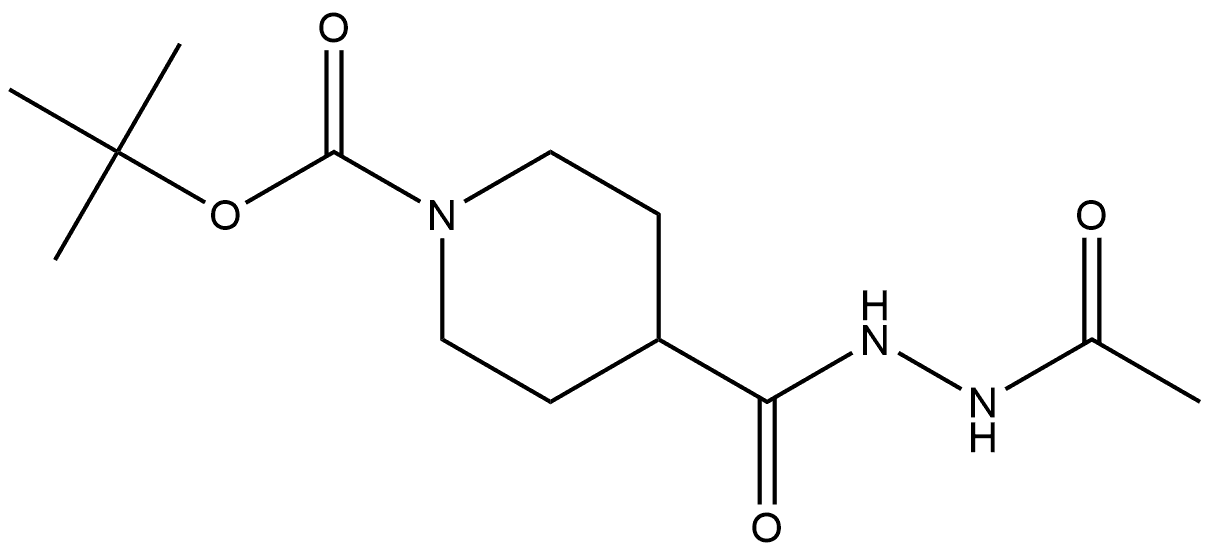
685827-77-4
0 suppliers
inquiry

161609-79-6
37 suppliers
$45.00/5mg
Yield:161609-79-6 83%
Reaction Conditions:
Stage #1: tert-butyl 4-(2-acetylhydrazinecarbonyl)piperidine-1-carboxylate.with iodine;triethylamine;triphenylphosphine in dichloromethane; for 2 h;Cooling with ice;
Stage #2: with trifluoroacetic acid in dichloromethane at 20;
Steps:
2-methyl-5-(4-piperidyl)-1,3,4-oxadiazole
To a flask is added triphenylphosphine (16.4 g, 61.9 mmol) and DCM (177 mL). The solution is stirred at RT and I2(16.0 g, 61.9 mmol) is added portion-wise; TEA (10.9 mL, 77.4 mmol) is added and the reaction mixture is stirred at RT for 15 min. The mixture is stirred in an ice-water bath and tert-butyl 4-(acetamidocarbamoyl)piperidine-l- carboxylate (9.3 g, 31.0 mmol) is added. The reaction mixture is stirred in an ice-water bath for 2 h, saturated aqueous NaHCO, solution is added, and the mixture is transferred to a separation funnel. The layers are separated and the aqueous layer is extracted with DCM. The combined organic extracts are dried over MgS04, filtered, and the filtrate is concentrated under reduced pressure to give a residue, which is dissolved in DCM (186 mL). To the solution is added TFA (46.5 mL) and the reaction mixture is stirred at RT overnight. The mixture is concentrated under reduced pressure and the resulting residue is combined with DCM and water. The layers are separated and the aqueous layer is extracted twice with EtOAc. The aqueous layer is basified to pH ~ 14 with 50% aqueous NaOH solution and extracted 6 times with DCM. The combined organic extracts are washed with saturated aqueous NaCl solution, dried over MgS04, and concentrated under reduced pressure to give a solid, which is dried under vacuum at 40 °C for 2 h, to obtain the title compound (4.3 g, 25.9 mmol, 83% yield) as an off-white solid. ES/MS m z: 168 (M+H).
References:
WO2019/245907,2019,A1 Location in patent:Paragraph 32-33
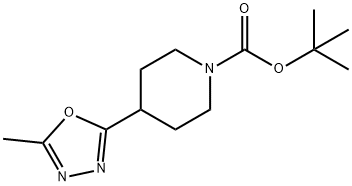
280110-69-2
43 suppliers
$26.00/250mg

161609-79-6
37 suppliers
$45.00/5mg
84358-13-4
391 suppliers
$5.00/5g

161609-79-6
37 suppliers
$45.00/5mg