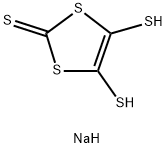
4,5-METHYLENEDITHIO-1,3-DITHIOLE-2-THIONE synthesis
- Product Name:4,5-METHYLENEDITHIO-1,3-DITHIOLE-2-THIONE
- CAS Number:70800-59-8
- Molecular formula:C4H2S5
- Molecular Weight:210.38
68494-08-6
52 suppliers
$26.57/1G

70800-59-8
32 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
with diiodomethane;sodium methylate in tetrahydrofuran;
Steps:
2
EXAMPLE 2 Example 2 also relates to an organic compound crystal according to the embodiment of the present invention and a FET according to the first embodiment of the present invention, and further relates to a FET according to the twelfth embodiment of the present invention.The organic compound crystal in Example 2 is composed of bis(methylenedithio)tetrathiafulvalene (BMDT-TTF) corresponding to a ?-electron conjugated molecule represented by structural formula (11') below which contains chalcogen atoms, i.e., sulfur (S), as a constituent. The FET in Example 2 has the same structure as that shown in FIG. 3A which is a schematic partial sectional view.In Example 2, the distance between chalcogen atoms (sulfur, S) of a ?-electron conjugated molecule (BMDT-TTF) and chalcogen atoms (sulfur, S) of its adjacent ?-electron conjugated molecule (BMDT-TTF) is short. That is, chalcogen atoms of a ?-electron conjugated molecule and chalcogen atoms of its adjacent ?-electron conjugated molecule are linked to each other. In other words, chalcogen atoms of a ?-electron conjugated molecule and the chalcogen atoms of its adjacent ?-electron conjugated molecule have interactions among each other. The organic compound crystal has a periodic structure in which ?-electron conjugated molecules are two-dimensionally linked together (namely, a two-dimensional network structure is formed).Specifically, sulfur (S), which is the chalcogen atom in Example 2, has the van der Waals' radius (r1=r2) of 1.80 as described in Example 1. Among chalcogen atoms (Xi) in a ?-electron conjugated molecule and chalcogen atoms (Xj) in its adjacent ?-electron conjugated molecule, the distance Rij between at least one pair of chalcogen atoms (Xi, Xj) satisfies the relationship Rij(r1+r2)×1.1=3.96 . The Rij value is, for example, 3.56 or 3.60 . Furthermore, sulfur (S), i.e., the chalcogen atom, is contained in the ?-electron conjugated system, conjugated with the ?-electron conjugated system, participating in the ?-electron conjugated system, or taken up by the ?-electron conjugated system. Furthermore, in each ?-electron conjugated molecule, sulfur (S), i.e., the chalcogen atom, is placed on the outer periphery of the ?-electron conjugated molecule. The ratio of the total mass of chalcogen atoms to the molecular weight of the ?-electron conjugated molecule (BMDT-TTF: 356.59) is 40% or more (in particular, 71.9%).A synthesis example of BMDT-TTF represented by structural formula (11'), single crystal structure analysis, and experimental fabrication of a thin-film transistor (TFT) for evaluation of physical properties will be described below.[Synthesis of BMDT-TTF]Synthesis was carried out according to Scheme 1 shown in FIG. 4. A compound (3), i.e., 4,5-bis(benzoylthio)-1,3-dithiol-2-one, was synthesized with reference to R. Schultz et al., J. Am. Chem. Soc. 105, 4519 (1983). The compound (3) in an amount of 1.95 g was heated together with 12 ml of P(OEt)3 in an argon atmosphere at about 110° C. As a result, dark red crystals were precipitated. The precipitate was separated by filtration, washed with ethanol, and dried in vacuum. Thereby, 0.80 g of BMDT-TTF was obtained. By recrystallization from CS2, a several millimeter-square thin-film single crystal was precipitated. With respect to BMDT-TTF, crystal structure analysis is reported in R. Kato et al., Chem. Lett. 1231 (1985). However, in order enhance the accuracy of data, the resulting BMDT-TTF single crystal was subjected to crystal structure analysis.[Single Crystal Structure Analysis of BMDT-TTF]Crystal structure analysis was carried out using the same single-crystal structure analyzer as that used in Example 1. Table 2 below shows the crystallographic data obtained. TABLE 2 Crystallographic data of BMDT-TTF Molecular formulaC8H4S8 Molecular weight 356.59 Measured temperature 90K X-ray wavelength used 0.71073 [] Crystal system monoclinic system Space groupP21/n Lattice constants of unit a-axis = 4.0972 (5) [] cell α = 90° b-axis = 24.233 (3) [] β = 105.060 (2)° c-axis = 6.2676 (8) [] γ = 90° Volume600.91 (13) [3] Z 2 Density (by calculation)1.971 g/cm3 FIGS. 5A and 5B show the crystal structure of BMDT-TTF. FIG. 5A is an a-axis projection in which the direction perpendicular to the sheet surface corresponds to the a-axis, and the vertical direction in the drawing corresponds to the c-axis. FIG. 5B is a c-axis projection in which the direction perpendicular to the sheet surface corresponds to the c-axis, and the vertical direction in the drawing corresponds to the a-axis. The crystal structure analysis shows that the BMDT-TTF molecules are stacked in the a-axis direction, and the strongest intermolecular interaction is likely to exist along the a-axis. Furthermore, interactions through the sulfur atoms on the outer peripheries are likely to occur along the c-axis. That is, a quasi-two-dimensional conduction band parallel to the (010) plane is expected. In order to perform quantitative evaluation, band analysis was carried out using ab-initio calculations.[Band Analysis of BMDT-TTF]Band analysis was carried out using an ab-initio calculation program, VASP (Vienna Ab-initio Simulation Package) (refer to G. Kresse and J. Furthmuller, Vienna Ab-initio Simulation Package, which is available from the website). Calculations were carried out using a density functional theory (DFT) method based on generalized gradient approximation (GGA). With respect to the lattice constants of the crystal and the coordinates of the individual atoms, the results of crystal structure analysis described above were used. The cut-off energy was set at 350 eV, and a self-consistent calculation was performed using a k-point mesh of 8×4×4. The results of band analysis are shown in FIG. 6.As is evident from FIG. 6, with respect to the conduction band including the highest occupied molecular orbit (HOMO) in the BMDT-TTF crystal, the interaction along the a-axis, which is the axis along which molecules are stacked, is strongest, with a dispersion of about 1 eV in terms of bandwidth. Furthermore, due to interactions of sulfur atoms placed parallel to the molecular plane, a dispersion of about 0.2 eV is also shown along the c-axis. The equi-energy cross section around the Γ point, which is the top of the HOMO band, clearly suggests the presence of a quasi-two-dimensional surface. Furthermore, as a result of evaluation of the effective mass m* along the a-axis in the conduction band of the BMDT-TTF crystal, m*=1.0 me. Here, me is the free electron mass. Consequently, the organic compound crystals composed of BMDT-TTF have inherent properties as a material for p-type channels superior to pentacene (effective mass: about 1.8 me).[Experimental Fabrication of Thin-Film Transistor (TFT) for Evaluation of Physical Properties]Using the BMDT-TTF thus obtained, a field-effect transistor (FET) including a thin-film transistor (TFT) was experimentally fabricated. Specifically, a gold electrode was disposed on the rear face of a highly doped silicon substrate to form a gate electrode. The front surface of the highly doped silicon substrate was subjected to thermal oxidation to form a gate insulating layer composed of SiO2 with a thickness of 100 nm. Comb-shaped source/drain electrodes were disposed on the gate insulating layer. The silicon substrate was dipped in a BMDT-TTF-saturated carbon disulfide solution, recovered from the solution, and naturally dried. Thereby, BMDT-TTF crystals were precipitated over the gate insulating layer and the source/drain electrodes. The electrical characteristics of the bottom gate/bottom contact type TFT thus fabricated were measured, and FIG. 7 shows the measurement results (Vd-Id characteristics with Vg as a parameter). As is evident from FIG. 7, it has been confirmed that BMDT-TTF also acts as a material for constituting the channel-forming region of the FET.
References:
US7425722,2008,B2 Location in patent:Page/Page column 12-14

68494-08-6
52 suppliers
$26.57/1G

75-11-6
341 suppliers
$10.00/5g

70800-59-8
32 suppliers
$45.00/10mg
59089-88-2
0 suppliers
inquiry

70800-59-8
32 suppliers
$45.00/10mg