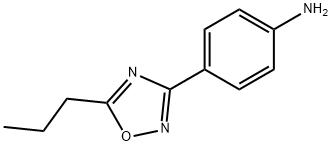
4-(5-Propyl-1,2,4-oxadiazol-3-yl)aniline synthesis
- Product Name:4-(5-Propyl-1,2,4-oxadiazol-3-yl)aniline
- CAS Number:10364-76-8
- Molecular formula:C11H13N3O
- Molecular Weight:203.24
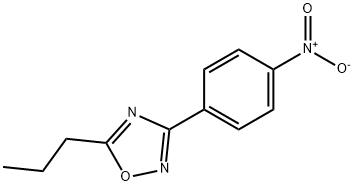
10364-67-7
13 suppliers
$60.00/50mg

10364-76-8
7 suppliers
$90.00/50mg
Yield:10364-76-8 54%
Reaction Conditions:
with disodium sulphide in 1,4-dioxane;lithium hydroxide monohydrate at 80; for 0.75 h;Inert atmosphere;
Steps:
19 3-(4′-Aminophenyl)-5-propyl-1,2,4-oxadiazole (19)
3.1.19
3-(4'-Aminophenyl)-5-propyl-1,2,4-oxadiazole (19)
A similar procedure
23
to that previously described for the preparation of 1 was followed using 5-propyl-3-(4-nitrophenyl)-1,2,4-oxadiazole (78) (0.34 g, 1.37 mmol) in dioxane (10 mL) and sodium sulfide (0.28 g, 3.55 mmol) in water (10 mL).
Purification by flash chromatography (hexane/ethyl acetate 3:2) afforded 19 as a yellow solid (0.16 g, 0.79 mmol, 54%). Rf = 0.80 (hexane/ethyl acetate, 1:1).
Mp 87-91 °C/[lit.
75
mp 88 °C]; 1H NMR (400 MHz; CDCl3) δH 1.04 (3H, t, J = 7.5 Hz, CH2CH2CH3), 1.89 (2H, m, CH2CH2CH3), 2.88 (2H, t, J = 7.5 Hz, CH2CH2CH3), 3.95 (2H, s, NH2), 6.72 (2H, d, J = 8.6 Hz, H-3', H-5') and 7.86 (2H, d, J = 8.6 Hz, H-2', H-6'); 13C NMR (100 MHz, CDCl3) δC 13.7 (CH3), 20.3 (CH2), 28.5 (CH2), 114.7 (CH), 116.8 (C), 128.9 (CH), 149.1 (C), 168.2 (C) and 179.3 (C); MS (ESI, 70 eV) m/z 204 (M+, 100%); Found (M+, 204.1133), C11H14N3O requires 204.1131.
References:
Conole, Daniel;Beck, Thorsten M.;Jay-Smith, Morgan;Tingle, Malcolm D.;Eason, Charles T.;Brimble, Margaret A.;Rennison, David [Bioorganic and medicinal chemistry]

555-16-8
530 suppliers
$5.00/5g

10364-76-8
7 suppliers
$90.00/50mg
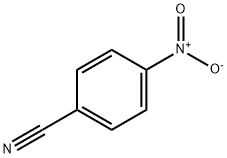
619-72-7
344 suppliers
$11.00/1g

10364-76-8
7 suppliers
$90.00/50mg
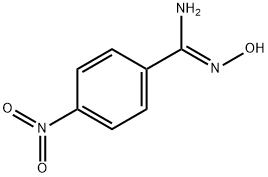
192332-48-2
2 suppliers
inquiry

10364-76-8
7 suppliers
$90.00/50mg