
4,6-Dimethyl-2(3H)-benzothiazolone synthesis
- Product Name:4,6-Dimethyl-2(3H)-benzothiazolone
- CAS Number:80567-67-5
- Molecular formula:C9H9NOS
- Molecular Weight:179.24
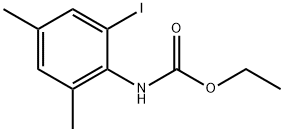
1373399-18-8
0 suppliers
inquiry

80567-67-5
15 suppliers
inquiry
Yield:80567-67-5 80%
Reaction Conditions:
Stage #1: ethyl 4,6-dimethyl-2-iodophenylcarbamatewith copper(l) iodide;sodiumsulfide nonahydrate in N,N-dimethyl-formamide at 80;Inert atmosphere;
Stage #2: with acetic acid in N,N-dimethyl-formamide at 130; for 36 h;Inert atmosphere;
Steps:
General procedure for the synthesis of benzothiazolone.
General procedure: A Schlenk tube was charged with ethyl 2-iodo-phenylcarbamates (1 mmol), CuI (19mg, 0.1 mmol), and Na2S*9H2O (3 mmol). The tube was evacuated and backfilled with argon (3 times), and the 2 mL of DMF was added. The reaction mixture wasstirred at 80 °C for 10-16 h, then 3 mL of AcOH was added to the cooled reaction mixture and the mixture was stirred at 130 °C for another 36 h. Saturated NaHCO3 (10mL) was added and the mixture was extracted with ethyl acetate. The organic layer was washed with H2O, brine, and dried over Na2SO4. After removal of the solvent in vacuo, the residue was purified by column chromatography on silica gel to provide the desired benzothiazolone.
References:
Li, Jiaojiao;Zhang, Yihua;Jiang, Yongwen;Ma, Dawei [Tetrahedron Letters,2012,vol. 53,# 20,p. 2511 - 2513] Location in patent:supporting information; experimental part