4,6-DINITRO INDOLE synthesis
- Product Name:4,6-DINITRO INDOLE
- CAS Number:245524-93-0
- Molecular formula:C8H5N3O4
- Molecular Weight:207.14
Yield:245524-93-0 70%
Reaction Conditions:
in acetic acid;
Steps:
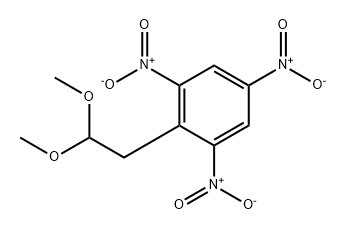
3 4,6-Dinitroindole
Example 3 4,6-Dinitroindole A solution of 1-(2,2-dimethoxy)ethyl-2,4,6-trinitrobenzene (117 mg, 0.388 mmol) in glacial acetic acid (1 ml) was refluxed and iron powder (69 mg, 1.17 mmol) was added. The heating source was removed and the exothermic reaction was allowed to subside. Then refluxing was continued for 30 minutes. The reaction mixture was cooled, poured into cold water, extracted with ethyl acetate, washed with water, brine and dried over anhydrous sodium sulfate. Removal of solvent gave 4,6-dinitroindole as a yellow solid (56 mg, 70%). 1 H NMR (Acetone d6): δ 7.28 (dd, H-2); 8.12 (d, 1H, H-3); 8.85 (m, 2H, H-5 & H-7). Mass: 208 (M+ +1).
References:
US5969155,1999,A
![1H-Indole, 2,3-dihydro-1-[(4-methylphenyl)sulfonyl]-4,6-dinitro-](/CAS/20210305/GIF/312513-83-0.gif)
312513-83-0
0 suppliers
inquiry

245524-93-0
27 suppliers
inquiry
296246-80-5
0 suppliers
inquiry

245524-93-0
27 suppliers
inquiry

2290-14-4
3 suppliers
inquiry

245524-93-0
27 suppliers
inquiry
35074-90-9
4 suppliers
inquiry

245524-93-0
27 suppliers
inquiry