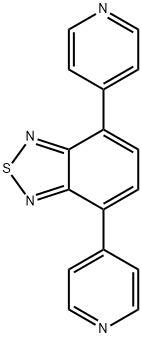
4,7-di(pyridin-4-yl)benzothiadiazole synthesis
- Product Name:4,7-di(pyridin-4-yl)benzothiadiazole
- CAS Number:692259-93-1
- Molecular formula:C16H10N4S
- Molecular Weight:290.34
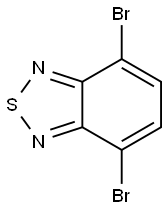
15155-41-6
318 suppliers
$21.00/1g
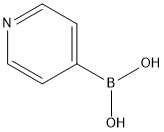
1692-15-5
442 suppliers
$6.00/1g

692259-93-1
19 suppliers
inquiry
Yield:692259-93-1 95%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in 1,4-dioxane;water at 90; for 2 h;Inert atmosphere;Suzuki Coupling;
Steps:
2.1.1. Preparation of compound 1
To a stirred solution of 4,7-Dibromo-2,1,3-benzothiadiazole (3.3 g, 11.2 mmol) in 1,4-dioxane (100 mL) was added pyridine- 4-boronic acid (3.44 g, 28 mmol), K 2 CO 3 (1.16g, 84 mmol) and tetrakis(triphenylphosphine)palladium (0.65 g, 0.56 mmol) at room temperature. The reaction mixture was stirred at 90 °C for 20 h under N 2 atmosphere, and then treated with water (50 mL), ex- tracted twice with CH 2 Cl 2 (40 mL ×2). The combined organic lay- ers were washed twice with water and once with brine, dried over anhydrous magnesium sulfate. After evaporation of the solvent un- der reduced pressure, The residue was purified by chromatography using DCM/EA (5/1, v/v) as an eluent to yield compound 1 as a yel- low solid (3.1 g, yiled: 95%). 1 H NMR (600 MHz, CDCl 3 ) 8.80 (d, J = 6.0 Hz, 4H), 7.93 (s, 6H). Anal. calcd for C 16 H 10 N 4 S: C, 66.19; H, 3.47; N, 19.30; found: C, 66.16; H, 3.50; N, 19.27.
References:
Chen, Hongjin;Ding, Zhonghua;Han, Yiying;Liu, Jian [Journal of Molecular Structure,2022,vol. 1262,art. no. 133073]

15155-41-6
318 suppliers
$21.00/1g
124252-41-1
76 suppliers
$24.00/250mg

692259-93-1
19 suppliers
inquiry