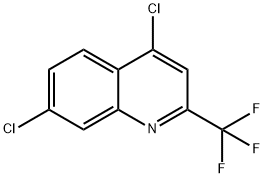
4,7-DICHLORO-2-(TRIFLUOROMETHYL)QUINOLINE synthesis
- Product Name:4,7-DICHLORO-2-(TRIFLUOROMETHYL)QUINOLINE
- CAS Number:702640-95-7
- Molecular formula:C10H4Cl2F3N
- Molecular Weight:266.05
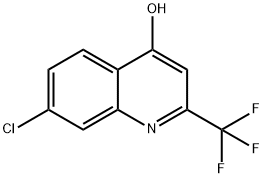
57124-20-6
72 suppliers
$70.00/250mg

702640-95-7
30 suppliers
$264.00/1g
Yield:-
Reaction Conditions:
with trichlorophosphate for 3 h;Heating / reflux;
Steps:
1.CC.a CC. 4-AMINOQUINOLINES
CC. 4-AMINOQUINOLINES (a) 4-Amino-7-chloro-2-trifluoromethylquinoline A mixture of 7-chloro-2-trifluoromethyl-4-quinolinol (4 g, 16.2 MMOL) in phosphorus oxychloride (7.55 mL, 81 MMOL) is heated at reflux temperature for 3 hours. After cooling to room temperature, ice (200 g) is added, the mixture is neutralized with NAHC03 AND extracted with EtOAc. The organic layer is washed with brine, dried (NA2SO4) and concentrated by rotary evaporator. The resulting tan residue is purified by flash chromatography on silica gel (1% ether in hexanes) to give 4, 7-DICHLORO-2- trifluoromethylquinoline as a solid. A solution of 4, 7-dichloro-2-trifluoromethylquinoline (2 g, 7.52 MMOL) in dioxane (10 mL) and NH3 (2 mL) are mixed AT-78°C in a sealed tube. After sealing and warming up to room temperature, the reaction is heated at 60-70°C for 19 hours and at 120°C for 3 hours. The sealed tube is cooled to-78°C before it is opened and the contents are concentrated by rotary evaporator. 4-Amino-7-chloro-2-trifluoromethylquinoline is obtained as a solid by trituration of the residue with ether. (b) 4-Amino-2, 7-BIS (trifluoromethyl) quinoline 4-Amino-2, 7-BIS (trifluoromethyl) quinoline is similarly prepared from 4-CHLORO-2, 7- BIS (TRIFLUOROMETHYL) QUINOLINE using ammonia in methanol and-is purified by flash chromatography on silica gel (10% EtOAc in hexanes). (c) 4-Amino-7-fluoroquinoline 4-Amino-7-fluoroquinoline is similarly prepared from 4-chloro-7-fluoroquinoline. (d) 4-Amino-6-fluoro-2- (trifluoromethyl) quinoline A mixture of 4-CHLORO-6-FLUORO-2- (TRIFLUOROMETHYL) QUINOLINE (1 g, 4.01 MMOL) and NH3 (3 mL) in ethylene glycol (20 mL) is prepared AT-78°C in a sealed tube. After warming up to room temperature, the sealed tube is gradually heated to 1 00°C overnight. After cooling to - 78°C, the sealed tube is opened and the contents are concentrated by rotary evaporator. The residue is purified by flash chromatography on silica gel (1: 3 EtOAc/hexanes) to give 4-AMINO-6-FLUORO-2- (TRIFLUOROMETHYL) QUINOLINE as a light yellow solid.
References:
WO2004/48314,2004,A1 Location in patent:Page 47-48