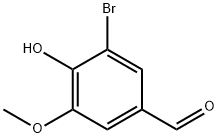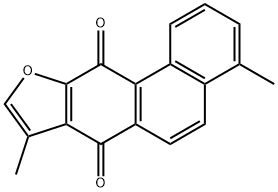
4,8-Dimethylphenanthro[3,2-b]furan-7,11-dione synthesis
- Product Name:4,8-Dimethylphenanthro[3,2-b]furan-7,11-dione
- CAS Number:20958-17-2
- Molecular formula:C18H12O3
- Molecular Weight:276.29

30684-15-2
0 suppliers
inquiry
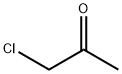
78-95-5
4 suppliers
$16.30/5 g
![4,8-Dimethylphenanthro[3,2-b]furan-7,11-dione](/CAS/GIF/20958-17-2.gif)
20958-17-2
31 suppliers
inquiry
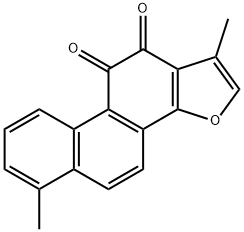
568-73-0
280 suppliers
$10.00/5mg
Yield: 12% , 12%
Reaction Conditions:
with ammonium acetate at 110; for 0.25 h;Sealed tube;Microwave irradiation;
Steps:
General representative procedure A for reactions with chloroacetone to form tanshinones1, 33, 35, 37, 39 and isotanshinones 32, 34, 36, 38, 40.
General procedure: A mixture of the alcohol 27-31 (100 mg, 0.42 mmol), ammonium acetate (32 mg, 0.42 mmol, 4 mmol g-1) and chloroacetone(10 mL g-1, 12 mmol) was heated in a sealed tube at 110 C for 15 minutes under microwaveconditions. The solution was cooled to room temperature, diluted with water (400 mLg-1) and extracted with DCM (3 x 400 mL g-1). The organic extracts were combined, dried(MgSO4), filtered and concentrated in vacuo to give the crude mixture which was purified byflash column chromatography (silica gel, DCM) to give the tanshinones 1, 33, 35, 37, 39 andisotanshinones 32, 34, 36, 38, 40. 1,6-Dimethylphenanthro[1,2-b]furan-10,11-dione, TI 1 and 4,8-dimethylphenanthro[3,2-b]furan-7,11-dione, iso-TI 32. General procedure A was followed, using the alcohol 27(100 mg, 0.42 mmol) to give the dione 1 (21 mg, 12% over 3 steps) as a dark red solid; mp 232-234 C (lit. [70] 229-230 C); δH(400 MHz; CDCl3) 9.23 (1 H, d, J 8.8, ArCH), 8.27 (1 H, d, J8.8, ArCH), 7.77 (1 H, d, J 8.8, ArCH), 7.54 (1 H, dd, J 8.8, 7.1, ArCH), 7.34 (1 H, d, J 7.1,ArCH), 7.30 (1 H, s, OCH), 2.68 (3 H, s, CH3), 2.30 (3 H, d, J 0.8, CH3); δC(100 MHz; CDCl3)183.4 (C = O), 175.6 (C = O), 161.2 (ArC), 142.0 (ArCH), 135.2 (ArC), 133.6 (ArC), 132.9(ArCH), 132.7 (ArC), 130.6 (ArCH), 129.6 (ArC), 128.3 (ArCH), 124.8 (ArCH), 123.1 (ArC),121.8 (ArC), 120.5 (ArC), 118.7 (ArCH), 19.9 (CH3), 8.8 (CH3); m/z (ESI+) 299 (10%, M+Na+),277 (100, M+H+). All data were in general agreement with the literature [66, 70, 71]. Alsoobtained was the dione 32 (22 mg, 12% over 3 steps) as an orange solid; mp 218-220 C (lit.[69] 219-220 C); max(ATR)/cm-1 1655 (C = O), 1588 (C = O), 1533 (C = C); δH(400 MHz;CDCl3) 9.66 (1 H, d, J 8.9, ArCH), 8.40 (1 H, dd, J 8.9, 0.8, ArCH), 8.32 (1 H, d, J 8.9, ArCH),7.63 (1 H, dd, J 8.9, 7.0, ArCH), 7.54-7.52 (1 H, m, OCH), 7.48 (1 H, dt, J 7.0, 0.8, ArCH), 2.76 (3 H, s, ArCH3), 2.41 (3 H, d, J 1.2, CH3); δC(101 MHz; CDCl3) 182.2 (C = O), 177.2 (C = O),153.9 (ArC), 145.2 (ArCH), 136.0 (ArC), 134.7 (ArC), 133.5 (ArC), 131.2 (ArC), 130.9 (ArCH),129.8 (ArCH), 129.3 (ArCH), 127.2 (ArC), 126.5 (ArC), 126.2 (ArCH), 122.1 (ArCH), 120.8(ArC), 20.0 (CH3), 8.7 (CH3); m/z (ESI+) 299 (25%, M+Na+), 277 (100, M+H+). No 13C NMRspectroscopy data were reported in the literature; all other data were in general agreementwith the literature [69, 70, 72].
References:
Foulkes, Matthew J.;Tolliday, Faith H.;Henry, Katherine M.;Renshaw, Stephen A.;Jones, Simon [PLoS ONE,2020,vol. 15,# 10 October,art. no. E0240231]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)